Summary
Nifedipine is a short-acting calcium antagonist formulated into several different oral preparations, each of which offers a distinct drug release profile. Of these, the nifedipine gastrointestinal therapeutic system (GITS) affords (rate)-controlled-release (CR) and once-daily administration. Although it is recognised that CR drug formulations may enhance the treatment compliance of patients by reducing the number of daily doses, there are several pharmaceutical, pharmacokinetic and pharmacological considerations which may influence the ultimate selection of a particular dosage form.
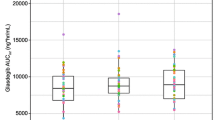
The formulation design of the nifedipine GITS involves an osmotic pump process which provides approximately zero-order delivery of the drug. This mechanism serves to prevent the possibility of dose dumping, but more importantly allows for maintenance of the relatively constant plasma drug concentrations assumed necessary to maintain smooth control of blood pressure. The pharmacokinetic characteristics of the nifedipine GITS have been evaluated in both single- and multiple-dose studies. The GITS formulation provides drug concentrations which reach a plateau within 6 hours after administration of a single dose, and continue at that concentration until at least 24 hours after administration. In this way large fluctuations in plasma drug concentrations are avoided, which may improve the efficacy and tolerability of the drug. Although a trend showing a small increase in the 24-hour plasma nifedipine concentrations has been observed by our group from some single-dose studies, it does not appear to be clinically relevant. One potentially important disadvantage of the GITS compared with ‘naturally’ long-acting agents is that the ‘intrinsic’ pharmacokinetic properties of nifedipine may be exposed in poorly compliant patients, leading to extended periods of subtherapeutic drug concentrations.
Drug delivery by the nifedipine GITS is unaffected by changes in pH and gastrointestinal (GI) motility, but the rate of drug release can increase slightly with food intake (although absolute bioavailability remains unchanged). No studies have been conducted to determine the average GI transit time of this particular dosage form, but it is possible that inadequate retention may occur in some patients, perhaps leading to less optimal clinical outcomes. For example, the median GI transit time for both oxprenolol and metoprolol Oros drug delivery systems has been reported as 27.4 hours, with individual times ranging from 5.1 to 58.3 hours. The possibility of inadequate GI retention of the nifedipine GITS is perhaps more likely in patients who have pre-existing GI motility disorders or who are taking other medications that enhance GI motility.
The interaction between grapefruit juice and nifedipine is interesting, considering that the exact mechanism involved has yet to be determined. Nonetheless, inhibition of presystemic metabolic processes (probably involving liver enzymes but possibly also enzymes contained within the wall of the small intestine) is likely to be a factor in the increased bioavailability of nifedipine observed in individuals coingesting grapefruit juice. Thus, potential nifedipine formulation differences with respect to the degree of interaction with grapefruit juice may occur if a significant degree of extrahepatic metabolism is involved.
The majority of clinical trials with the nifedipine GITS have assessed its efficacy in patients with mild-to-moderate essential hypertension, and have found it to be at least equivalent to other dosage forms of the drug. Since there is limited information available directly comparing the efficacy and adverse effects of the different types of nifedipine formulation, little attention has been focused on this subject. However, modifying the rate and duration of nifedipine release may profoundly affect the clinical performance of this drug. A slower rate of intravenous nifedipine infusion has been shown to reduce the incidence of vasodilator-related adverse effects and to improve blood pressure control. Therefore, these advantages may also apply to reduced rates of oral nifedipine absorption. Another important advantage of the nifedipine GITS is that the trough/peak effect ratio following once-daily administration is maintained above a value of 0.5 to 0.66, as is now typically suggested for antihypertensive therapy.
Similar content being viewed by others
References
Sorkin EM, Clissold SP, Brogden RN. Nifedipine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy, in ischaemic heart disease, hypertension and related cardiovascular disorders. Drugs 1985; 30: 182–274
Singh BN. Calcium channel blockade and cardiovascular therapeutics, In: Parmley WW, Chatterjee K, editors. Cardiovascular pharmacology. London: Wolfe, 1994: 6.1–6.27
Reicher-Reiss H, Barasch E. Calcium antagonists in patients with heart failure: a review. Drugs 1991; 42(3): 343–64
Lichtlen PR, Hugenholtz PG, Rafflenbeul W, et al. Retardation of angiographic progression of coronary artery disease by nifedipine. Results of the International Nifedipine Trial on Anti-atherosclerotic Therapy (INTACT). Lancet 1990; 335(8698): 1109–13
Eranko PO, Palva ES, Konno K, et al. Regulatory control of synonym drug bioavailability: bioinequivalency of nifedipine preparations. Pharm Med 1990; 4: 197–205
Kleinbloesem CH, van Brummelen P, van de Linde JA, et al. Nifedipine: kinetics and dynamics in healthy subjects. Clin Pharmacol Ther 1984; 35(6): 742–9
Foster TS, Hamann SR, Richards VR, et al. Nifedipine kinetics and bioavailability after single intravenous and oral doses in normal subjects. J Clin Pharmacol 1983; 23: 161–70
Kleinbloesem CH, van Brummelen P, Breimer DD. Nifedipine: relationship between pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 1987; 12: 12–29
Kleinbloesem CH, van Brummelen P, Danhof M, et al. Rate of increase in the plasma concentration of nifedipine as a major determinant of its hemodynamic effects in humans. Clin Pharmacol Ther 1987; 41(1): 26–30
Steinijans VW. Pharmacokinetic characterization of controlled-release formulations. Eur J Drug Metab Pharmacokinet 1990; 15(2): 173–81
Devane JG, Mulligan S, Kavanagh M, et al. New developments in sustained-release antihypertensive therapy: formulation and pharmacokinetic considerations. Am J Cardiol 1992; 69: 23E-27E
van Zwieten PA, Pfaffendorf M. Pharmacology of the dihydropyridine calcium antagonists: relationship between lipophilicity and pharmacodynamic responses. J Hypertens 1993; 11 Suppl. 6: S3–8
Prisant LM, Bottini B, DiPiro JT, et al. Novel drug-delivery systems for hypertension. Am J Med 1992; 93 Suppl. 2A: 45S–55S
Avgerinos A, Gorrod JW. Pharmacokinetics of nifedipine derived from a new retard tablet formulation. Eur J Drug Metab Pharmacokinet 1990; 15(4): 273–8
Pabst G, Lutz D, Molz KH, et al. Pharmacokinetics and bioavailability of three different galenic nifedipine preparations. Arzneim Forsch 1986; 36(1): 256–60
Swanson DR, Barclay BL, Wong PS, et al. Nifedipine gastrointestinal therapeutic system. Am J Med 1987; 83 Suppl. 6B: 3–9
Kelly JG, O’Malley K. Clinical pharmacokinetics of calcium antagonists: an update. Clin Pharmacokinet 1992; 22(6): 416–33
Michelson EL. Calcium antagonists in cardiology: update on sustained-release drug delivery systems. Clin Cardiol 1991; 14: 947–50
Data on file. Etobicoke, Ontario: Bayer Canada Inc., 1995
Santus G, Baker RW. Osmotic drug delivery: a review of the patent literature. J Control Release 1995; 35: 1–21
Kellaway IW. Scientific rationale and clinical implications of sustained-release formulations. Br J Clin Pract 1988; 60 Symp. Suppl.: 9–13
Theeuwes F. Elementary osmotic pump. J Pharm Sci 1975; 64(12): 1987–91
Chung M, Reitberg DP, Gaffney M, et al. Clinical pharmacokinetics of nifedipine gastrointestinal therapeutic system. A controlled-release formulation of nifedipine. Am J Med 1987; 83 Suppl. 6B: 10–4
Qureshi SA, Caillé G, Brien R, et al. Application of flow-through dissolution method for the evaluation of oral formulations of nifedipine. Drug Dev Ind Pharm 1994; 20(11): 1869–82
Grundy JS, Foster RT, Grace M, et al. A single dose pharmacokinetic (PK) comparison of prolonged-action (PA) and gastrointestinal therapeutic system (GITS) nifedipine (NIF) in elderly volunteers [abstract]. Pharmaceut Res 1994; 11(10): S–401
Crome P, Muller FO, Wijayawardhana P, et al. Single dose and steady-state pharmacokinetic profiles of nifedipine GITS tablets in healthy elderly and young volunteers. Drug Invest 1993; 5(4): 193–9
Schneider R, Stolero D, Griffel L, et al. Pharmacokinetic profile of nifedipine GITS in hypertensive patients with chronic renal impairment. Drugs 1994; 48 Suppl. 1: 16–22
Raemsch KD, Sommer J. Pharmacokinetics and metabolism of nifedipine. Hypertension 1983; 5 (4 Suppl. 2): 19–24
Soons PA, Schoemaker HC, Cohen AF, et al. Intraindividual variability in nifedipine pharmacokinetics and effects in healthy subjects. J Clin Pharmacol 1992; 32: 324–31
Reitberg DP, Love SJ, Quercia GT, et al. Effect of food on nifedipine pharmacokinetics. Clin Pharmacol Ther 1987; 42(1): 72–5
Challenor V, Waller DG, Gruchy BS, et al. The effects of food and posture on the pharmacokinetics of a biphasic release preparation of nifedipine. Br J Clin Pharmacol 1986; 22: 565–70
Myers MG, Raemsch KD. Comparative pharmacokinetics and antihypertensive effects of the nifedipine tablet and capsule. J Cardiovasc Pharmacol 1987; 10 Suppl. 10: S76–8
Ahsan CH, Renwick AG, Macklin B, et al. Ethnic differences in the pharmacokinetics of oral nifedipine. Br J Clin Pharmacol 1991; 31: 399–403
Qureshi S, Laganiere S, Caillé G, et al. Effect of an acute dose of alcohol on the pharmacokinetics of oral nifedipine in humans. Pharm Res 1992; 9(5): 683–6
Robertson DR, Waller DG, Renwick AG, et al. Age-related changes in the pharmacokinetics and pharmacodynamics of nifedipine. Br J Clin Pharmacol 1988; 25: 297–305
Taburet AM, Singlas E, Colin JN, et al. Pharmacokinetic studies of nifedipine tablet. Correlation with antihypertensive effects. Hypertension 1983; 5 (4 Suppl. 2): 29–33
Scott M, Castleden CM, Adam HK, et al. The effect of ageing on the disposition of nifedipine and atenolol. Br J Clin Pharmacol 1988; 25: 289–96
Sigusch H, Hippius M, Henschel L, et al. Influence of grapefruit juice on the pharmacokinetics of a slow release nifedipine formulation. Pharmazie 1994; 49: 522–4
Hoyo-Vadillo C, Castañeda-Hernández G, Herrera JE, et al. Pharmacokinetics of nifedipine slow release tablet in Mexican subjects: further evidence for an oxidation polymorphism. J Clin Pharmacol 1989; 29: 816–20
Akopov SE, Sarkissian MA, Grigorian GS, et al. Nifedipine kinetics and dynamics in hypertensive patients: a new approach. Int J Clin Pharmacol Ther 1994; 32(6): 317–20
Ueno K, Kawashima S, Uemoto K, et al. Effect of food on nifedipine sustained-release preparation. DICP 1989; 23: 662–5
Grundy JS, Foster RT, du Souich P, et al. A single-dose pharmacokinetic (PK) comparison of three prolonged-action (PA) nifedipine (NIF) formulations [abstract]. Pharm Res 1994; 11(10): S401
Groenewoud G, Luus HG, Müller FO, et al. Pharmacokinetics of nifedipine GITS after single dose and at steady-state in healthy elderly and young subjects [abstract]. S Afr Med J 1991; 80: 623
Khan A, Langley SJ, Mullins FG, et al. The pharmacokinetics and pharmacodynamics of nifedipine at steady state during concomitant administration of cimetidine or high dose ranitidine. Br J Clin Pharmacol 1991; 32: 519–22
Debbas NM, Jackson SH, Shah K, et al. The bioavailability and pharmacokinetics of slow release nifedipine during chronic dosing in volunteers. Br J Clin Pharmacol 1986; 21: 385–8
Schall R, Muller FO, Hundt HK, et al. Relative bioavailability of four controlled-release nifedipine products. Biopharm Drug Dispos 1994; 15: 493–503
Guengerich FP, Brian WR, Iwasaki M, et al. Oxidation of dihydropyridine calcium channel blockers and analogues by human liver cytochrome P-450 IIIA4. J Med Chem 1991; 34: 1838–44
Guengerich FP, Martin MV, Beaune PH, et al. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J Biol Chem 1986; 261(11): 5051–60
Krishna DR, Klotz U. Extrahepatic metabolism of drugs in humans. Clin Pharmacokinet 1994; 26(2): 144–60
Edgar B, Bailey DG, Bergstrand R, et al. Formulation dependent interaction between felodipine and grapefruit juice [abstract]. Clin Pharmacol Ther 1990; 47(2): 181
Challenor VF, Waller DG, Renwick AG, et al. The trans-hepatic extraction of nifedipine. Br J Clin Pharmacol 1987; 24: 473–7
Murdoch D, Brogden RN. Sustained release nifedipine formulations: an appraisal of their current uses and prospective roles in the treatment of hypertension, ischaemic heart disease and peripheral vascular disorders [published erratum appears in Drugs 1991 Aug; 42 (2): 330]. Drugs 1991; 41: 737–79
Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Marcel Dekker Inc., 1982
Boxenbaum H. Pharmacokinetic determinants in the design and evaluation of sustained-release dosage forms. Pharm Res 1984; 82–8
Ilett KF, Tee LBG, Reeves PT, et al. Metabolism of drugs and other xenobiotics in the gut lumen and wall. Pharmacol Ther 1990; 46: 67–93
de Boer AG, Moolenaar F, de Leede LGJ, et al. Rectal drug administration: clinical pharmacokinetic considerations. Clin Pharmacokinet 1982; 7: 285–311
Lemmer B, Bruguerolle B. Chronopharmacokinetics: are they clinically relevant? Clin Pharmacokinet 1994; 26: 419–27
Lemmer B, Scheidel B, Behne S. Chronopharmacokinetics and chronopharmacodynamics of cardiovascular active drugs. Propranolol, organic nitrates, nifedipine. Ann NY Acad Sci 1991; 618: 166–81
Lemmer B, Nold G, Behne S, et al. Chronopharmacokinetics and cardiovascular effects of nifedipine. Chronobiol Int 1991; 8: 485–94
Lemmer B. The cardiovascular system and daily variation in response to antihypertensive and antianginal drugs: recent advances. Pharmacol Ther 1991; 51: 269–74
Shargel L, Yu ABC. Applied biopharmaceutics and pharmacokinetics. 3rd ed. Norwalk (CN): Appleton and Lange, 1993
Castañeda-Hernández G, Caille G, du Souich P. Influence of drug formulation on drug concentration-effect relationships. Clin Pharmacokinet 1994; 26(2): 135–43
Castañeda-Hernández G, Hoyo-Vadillo C, Herrera JE. Differences in nifedipine concentration-effect relationship between capsule and slow release tablet administration. Int J Clin Pharmacol Ther 1995; 33(1): 56–60
Myers MG, Tanner J, Leenen FHH, et al. Comparative efficacy of two long-acting formulations of nifedipine in the treatment of hypertension [abstract]. Presented at the 9th Scientific Meeting of the American Society of Hypertension, 1994
Davis SS, Hardy JG, Taylor MJ, et al. The in-vivo evaluation of an osmotic device (Osmet) using gamma scintigraphy. J Pharm Pharmacol 1984; 36: 740–2
Mojaverian P, Chan K, Desai A, et al. Gastrointestinal transit of a solid indigestible capsule as measured by radiotelemetry and dual gamma scintigraphy. Pharm Res 1989; 6(8): 719–24
Wilson CG, Hardy JG. Gastrointestinal transit of an osmotic tablet drug delivery system. J Pharm Pharmacol 1985; 37: 573–5
Davis SS, Washington N, Parr GD, et al. Relationship between the rate of appearance of oxprenolol in the systemic circulation and the location of an oxprenolol Oros 16/260 drug delivery system within the gastrointestinal tract as determined by scintigraphy. Br J Clin Pharmacol 1988; 26: 435–43
John VA, Shotton PA, Moppert J, et al. Gastrointestinal transit of Oros drug delivery systems in healthy volunteers: a short report. Br J Clin Pharmacol 1985; 19 Suppl. 2: 203S–206S
Hirtz J. The absorption window: fact or fiction? Pharm Int 1984; 175–8
Hardy JG, Wilson CG, Wood E. Drug delivery to the proximal colon. J Pharm Pharmacol 1985; 37: 874–7
Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology 1987; 92: 40–7
Prisant LM, Carr AA, Bottini PB, et al. Nifedipine GITS (gastrointestinal therapeutic system) bezoar. Arch Intern Med 1991; 151: 1868–9
Greenstein DB, Wilcox CM, Frontin K, et al. Nifedipine (Procardia XL) as a cause of false-positive results on barium enema study. South Med J 1994; 87(8): 808–10
Adalat XL® Product Monograph. Etobicoke, Ontario: Bayer Canada Inc., 1995
Ritschel WA. Diseases and disease states that influence gastrointestinal absorption of drugs. J Pharmaceut Sci 1993; 82(9): 862–5
Bailey DG, Arnold JM, Spence JD. Grapefruit juice and drugs: How significant is the interaction? Clin Pharmacokinet 1994; 26(2): 91–8
Bailey DG, Spence JD, Munoz C, et al. Interaction of citrus juices with felodipine and nifedipine. Lancet 1991; 337: 268–9
Rashid J, Mckinstry C, Renwick AG, et al. Quercetin, an in vitro inhibitor of CYP3A, does not contribute to the interaction between nifedipine and grapefruit juice. Br J Clin Pharmacol 1993; 36: 460–3
Tarn YK. Individual variation in first-pass metabolism. Clin Pharmacokinet 1993; 25(4): 300–28
Bailey DG, Arnold MO, Bend JR, et al. Grapefruit juice-felodipine interaction: reproducibility and characterization with the extended release drug formulation. Br J Clin Pharmacol 1995; 40: 135–40
Graefe KH, Ziegler R, Wingender W, et al. Plasma concentration-response relationships for some cardiovascular effects of dihydropyridines in healthy subjects. Clin Pharmacol Ther 1988; 43(1): 16–22
Opie LH. Calcium antagonists. Mechanisms, therapeutic indications and reservations: a review. Q J Med 1984; NS LIII (209): 1–16
Zsotée TT, Church JG. Calcium antagonists. Pharmacodynamic effects and mechanism of action. Drugs 1983; 25: 93–112
Borchard U. Calcium antagonists in comparison: view of the pharmacologist. J Cardiovasc Pharmacol 1994; 24 Suppl. 2: S85–91
Frishman WH, Garofalo JL, Rothschild A, et al. The nifedipine gastrointestinal therapeutic system in the treatment of hypertension. Am J Cardiol 1989; 64: 65F-69F
Frohlich ED, McLoughlin MJ, Losem CJ, et al. Hemodynamic comparison of two nifedipine formulations in patients with essential hypertension. Am J Cardiol 1991; 68: 1346–50
Hilleman DE, Mohiuddin SM, Lucas Jr BD, et al. Conversion from sustained-release to immediate-release calcium entry blockers: outcome in patients with mild-to-moderate hypertension. Clin Ther 1993; 15(6): 1002–10
Vetrovec GW, Parker VE, Cole S, et al. Nifedipine gastrointestinal therapeutic system in stable angina pectoris. Results of a multicenter open-label crossover comparison with standard nifedipine. Am J Med 1987; 83 Suppl. 6B: 24–9
Hosie J, Bremner AD, Fell PJ, et al. Side effects of dihydropyridine therapy: comparison of amlodipine and nifedipine retard. J Cardiovasc Pharmacol 1993; 22 Suppl. A: S9–12
Galletti F, Barba G, Nardecchia A, et al. Controlled study with a new sustained-release formulation of nifedipine in essential hypertensive patients. J Clin Pharmacol 1994; 34: 919–23
Rogstad B. A comparison of lisinopril and nifedipine in the treatment of mild to moderate hypertension. A multicentre study. Eur J Clin Pharmacol 1994; 46: 487–9
Mörlin C, Baglivo H, Boeijinga JK, et al. Comparative trial of lisinopril and nifedipine in mild to severe essential hypertension. J Cardiovasc Pharmacol 1987; 9 Suppl. 3: S49–52
Feig PU, Gibson L, Mac Carthy EP, et al. The efficacy and safety of once-daily nifedipine coat-core in the treatment of mild-to-moderate hypertension. Adalat CC Cooperative Study Group [published erratum appears in Clin Ther 1994 Jan-Feb; 16 (1): 125]. Clin Ther 1993; 15(6): 963–75
Glasser SP, Jain A, Allenby KS, et al. The efficacy and safety of once-daily nifedipine: the coat-core formulation compared with the gastrointestinal therapeutic system formulation in patients with mild-to-moderate diastolic hypertension. Nifedipine Study Group. Clin Ther 1995; 17(1): 12–29
Fagan TC, Haggert BE, Liss C. Efficacy and tolerability of extended-release felodipine and extended-release nifedipine in patients with mild-to-moderate essential hypertension. Clin Ther 1994; 16: 634–46
Frishman WH, Garofalo JL, Rothschild A, et al. Multicenter comparison of the nifedipine gastrointestinal therapeutic system and long-acting propranolol in patients with mild to moderate systemic hypertension receiving diuretics. A preliminary experience. Am J Med 1987; 83 Suppl. 6B: 15–9
Gavras I, Mulinari R, Gavras H, et al. Antihypertensive effectiveness of the nifedipine gastrointestinal therapeutic system. Am J Med 1987; 83 Suppl. 6B: 20–3
Krakoff LR, Bravo EL, Tuck ML, et al. Nifedipine gastrointestinal therapeutic system in the treatment of hypertension. Results of a multicenter trial. The Modern Approach to the Treatment of Hypertension (MATH) Study Group. Am J Hypertens 1990; 3 (12 Pt 2): 318S–327S
Murphy C, McNamara C, Mohanty PK. The antihypertensive effects of nifedipine (GITS) in mild to moderate hypertension [abstract]. Am J Hypertens 1989; 2 (5 Pt 2): 28A
Lewis GP, Ames RP, Halperin AK, et al. Nifedipine gastrointestinal therapeutic system (NGITS) in patients with mild essential hypertension: therapeutic effects of a novel delivery system [abstract]. Am J Hypertens 1989; 2 (5 Pt 2): 18A
Houston MC, Olafsson L, Burger MC. Effects of nifedipine GITS and atenolol monotherapy on serum lipids, blood pressure, heart rate, and weight in mild to moderate hypertension. Angiology 1991; 42(9): 681–90
Zanchetti A, Bianchi L, Amigoni S, et al. Antihypertensive effects of nifedipine gastrointestinal therapeutic system on clinic and ambulatory blood pressure in essential hypertensives. J Hypertens 1993; 11 Suppl. 5: S334–5
Zanchetti A. The 24-hour efficacy of a new once-daily formulation of nifedipine. Italian Nifedipine GITS Study Group. Drugs 1994; 48 Suppl. 1: 23–30
Tuck ML, Bravo EL, Krakoff LR, et al. Endocrine and renal effects of nifedipine gastrointestinal therapeutic system in patients with essential hypertension. Results of a multicenter trial. The Modern Approach to the Treatment of Hypertension Study Group. Am J Hypertens 1990; 3 (12 Pt 2): 333S–341S
Krakoff LR. Effectiveness of nifedipine gastrointestinal therapeutic system for treatment of hypertension: results of the MATH Trial. J Cardiovasc Pharmacol 1993; 21 Suppl. 2: S14–7
Zanchetti A. Short- and long-term perspectives of antihypertensive therapy. An introduction. Am J Hypertens 1993; 6 (3 Pt 2): 2S–5S
Mancia G, Frattola A, Omboni S, et al. Twenty-four-hour blood pressure and antihypertensive treatment. Blood Press 1992; Suppl. 1: 44–6
Meredith PA, Elliott HL. FDA guidelines on trough: peak ratios in the evaluation of antihypertensive agents. J Cardiovasc Pharmacol 1994; 23 Suppl. 5: S26–30
Donnelly R, Elliott HL, Meredith PA. Concentration-effect analysis of antihypertensive drug response. Focus on calcium antagonists. Clin Pharmacokinet 1994; 26(6): 472–85
Meredith PA, Donnelly R, Elliott HL, et al. Prediction of the antihypertensive response to enalapril. J Hypertens 1990; 8: 1085–90
Bainbridge AD, Herlihy O, Meredith PA, et al. A comparative assessment of amlodipine and felodipine ER: pharmacokinetic and pharmacodynamic indices. Eur J Clin Pharmacol 1993; 45: 425–30
Glasser SP, Ripa SR, Allenby KS, et al. The efficacy and safety of once-daily nifedipine administered without food: the coat-core formulation compared with the gastrointestinal therapeutic system formulation in patients with mild-to-moderate hypertension. Clin Ther 1995; 17(2): 296–312
Echizen H, Eichelbaum M. Clinical pharmacokinetics of verapamil, nifedipine and diltiazem. Clin Pharmacokinet 1986; 11: 425–49
Meredith PA, Elliott HL, Donnelly R, et al. Dose-response clarification in early drug development. J Hypertens 1991; 9 Suppl. 6: S356–7
Donnelly R, Elliott HL, Meredith PA, et al. Nifedipine: individual responses and concentration-effect relationships. Hypertension 1988; 12(4): 443–9
Banerjee PS, Robinson JR. Novel drug delivery systems: an overview of their impact on clinical pharmacokinetic studies. Clin Pharmacokinet 1991; 20(1): 1–14
Francheteau P, Steimer JL, Merdjan H, et al. A mathematical model for dynamics of cardiovascular drug action: application to intravenous dihydropyridines in healthy volunteers. J Pharmacokinet Biopharmaceut 1993; 21(5): 489–514
Castañeda-Hernández G, Hoyo C, Herrera JE, et al. Comparacion farmacocinetica de dos formulaciones orales de nifedipina: capsula de 10 mg y tableta de liberacion pro-longada de 20 mg [abstract]. Memorias del XV Congreso Nacional de Cardiololgia, Villahermosa, Mexico. 1987; 214
Breimer DD, Urquhart J. Nifedipine GITS [correspondence]. Lancet 1993; 341: 306
Nifedipine: a new life with GITS? [editorial]. Lancet 1992; 340: 1507–8
Nayler WG. Pharmacological aspects of calcium antagonism: short term and long term benefits. Drugs 1993; 46 Suppl. 2: 40–7
Sewester CS, Olin BR, Hebel SK, et al. Facts and comparisons. New York: J.B. Lippincott Co., 1991
Opie LH, White DA. Adverse interaction between nifedipine and beta-blockade. Br Med J 1980; 281(6253): 1462
Monsen L, Moisey D, Gaffney M, et al. Consistent blood pressure reduction without loss of diurnal variability with once-daily nifedipine GITS treatment [abstract]. Am J Hypertens 1990; 3 (5 Pt 2): 114A
Geizhals M, Phillips RA, Ardeljan M, et al. Sustained calcium channel blockade in the treatment of severe hypertension. A two year experience. Am J Hypertens 1990; 3: 313S–317S
Martsevich SY, Metelitsa VI, Rumiantsev DO, et al. Development of tolerance to nifedipine in patients with stable angina pectoris. Br J Clin Pharmacol 1990; 29: 339–46
Salvetti A, Di Venanzio L. Clinical pharmacology of long-acting calcium antagonists: what relevance for therapeutic effects? J Cardiovasc Pharmacol 1994; 23 Suppl. 5: S31–4
Oosterhuis B, Jonkman JH. Advantages and disadvantages of reformulation of older drugs. Clin Pharmacokinet 1993; 25(3): 165–71
Elliott HL, Meredith PA. Nifedipine GITS [correspondence]. Lancet 1993; 341: 306
Rudd P, Lenert L. Pharmacokinetics as an aid to optimising compliance with medications. Clin Pharmacokinet 1995; 28(1): 1–6
Author information
Authors and Affiliations
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/BF03257496.
Rights and permissions
About this article
Cite this article
Grundy, J.S., Foster, R.T. The Nifedipine Gastrointestinal Therapeutic System (GITS). Clin-Pharmacokinet 30, 28–51 (1996). https://doi.org/10.2165/00003088-199630010-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199630010-00003