Abstract
Diacerein is a drug for the treatment of patients with osteoarthritis. This drug is administered orally as 50mg twice daily. Diacerein is entirely converted into rhein before reaching the systemic circulation. Rhein itself is either eliminated by the renal route (20%) or conjugated in the liver to rhein glucuronide (60%) and rhein sulfate (20%); these metabolites are mainly eliminated by the kidney.
The pharmacokinetics characteristics of diacerein are about the same in young healthy volunteers and elderly people with normal renal function, both after a single dose (50mg) or repeated doses (25 to 75mg twice daily). Rhein kinetics after single oral doses of diacerein are linear in the range 50 to 200mg. However, rhein kinetics are time-dependent, since the nonrenal clearance decreases with repeated doses. This results in a moderate increase in maximum plasma concentration, area under the plasma concentration-time curve and elimination half-life. Nevertheless, the steady-state is reached by the third administration and the mean elimination half-life is then around 7 to 8 hours.
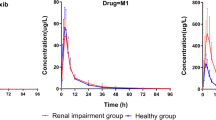
Taking diacerein with a standard meal delays systemic absorption, but is associated with a 25% increase in the amount absorbed. Mild-to-severe (Child Pugh’s grade B to C) liver cirrhosis does not change the kinetics of diacerein, whereas mild-to-severe renal insufficiency (creatinine clearance <2.4 L/h) is followed by accumulation of rhein which justifies a 50% reduction of the standard daily dosage.
Rhein is highly bound to plasma proteins (about 99%), but this binding is not saturable so that no drug interactions are likely to occur, in contrast to those widely reported with nonsteroidal anti-inflammatory drugs. Except for moderate and transient digestive disturbances (soft stools, diarrhoea), diacerein is well tolerated and seems neither responsible for gastrointestinal bleeding nor for renal, liver or haematological toxicity.
Similar content being viewed by others
References
Spencer CM, Wilde MI. Diacerein. Drugs 1997 Jan; 53 (1): 98–106.
Franchi-Micheli S, Lavacchi L, Friedmann CA, et al. The influence of rhein on the biosynthesis of prostaglandin-like substances in-vitro. J Pharm Pharmacol 1983; 35: 262–4.
Pomarelli P, Berti M, Gatti MT, et al. A non steroidal anti-inflammatory drug that stimulates prostaglandin release. Farmaco [Sci] 1980; 35: 836–42.
La Villa G, Marra F, Laffi G, et al. Effects of rhein on renal arachidonic acid metabolism and renal function in patients with congestive heart failure. Eur J Clin Pharmacol 1989; 37: 1–5.
Dougados M. Diacerein: a viewpoint. Drugs 1997 Jan; 53 (1): 107.
Benetti D, Mian M. Diacerein: a viewpoint. Drugs 1997 Jan; 53 (1): 107–8.
Mazieres B. Diacerein: a viewpoint. Drugs 1997 Jan; 53 (1): 108.
Dougados M, Nguyen M, Berdah L, et al. Méthodes d’évaluation de l’arthrose: à propos de l’étude ECHODIAH. Rev Prat 1996 Mar 15; 46 Suppl. 6: S53–6.
Springolo V, Coppi G. Simple method for the determination of rhein in biological fluids by HPLC. J Chromatogr 1988; 428: 173–7.
Debord P, Louchahi K, Tod M, et al. Influence of renal function on the pharmacokinetics of diacerein after a single oral dose. Fundam Clin Pharmacol 1993; 7: 435–41.
Uchino K, Yamamura Y, Saitoh Y, et al. Determination of rhein and its conjugates in urine by HPLC. J Chromatogr 1986; 380: 462–7.
Magnard O, Louchahi K, Tod M, et al. Pharmacokinetics of diacerein in patients with liver cirrhosis. Biopharm Drug Dispos 1993; 14: 401–8.
Petitjean O, Nicolas P, Louchahi K, et al. Etude de la pharmacocinétique de la diacereine en administration aiguë orale à la dose de 50mg à jeun et au cours d’un repas puis en administration réitérée à la dose de 50 × 2 mg/jour chez le sujet non pathologique adulte. Laboratoires NEGMA (Study report); Study PC/ART 9026 N; 1991 Apr.
Weyhenmeyer R, Kramarczyk R, Daak EV, et al. Study on doselinearity of diacerein in healthy volunteers. Laboratoires NEGMA-MADAUS (Study Report); Study DA 39WO.06B; 1990 Nov.
De Witte P, Lemli J. Metabolism of 14C-rhein and 14C-rhein anthrone in rats. Pharmacology 1988; 36 Suppl. 1: 152–7.
Barre J, Ledudal P, Tillement JP Etude ex vivo de l’incidence de l’insuffisance rénale chronique et de la cirrhose sur la fixation plasmatique de la rhéine, métabolite actif de la diacétylrhéine (diacerein). Laboratoires NEGMA, Study PC/ART 90–39 N, Study report, Jan 1991.
Segré G. Etude pilote du passage de la rhéine dans le liquide synovial. Laboratoires NEGMA, Study 4A29, Study report, Jul 1988.
Berdah L, Bono I, Bodinier MC, et al. Effets du métabolite actif de la diacerhéine sur la production d’IL1β par les monocytes humains en culture [abstract D23]. Rev Rhum Ed Fr 1993; 60: 678.
Boittin M, Redini F, Loyau G, et al. Effet de la diacerhéine (ART 50®) sur la synthèse de la matrice et la sécrétion de collagénase par des chondrocytes articulaires de lapin en culture. Rev Rhum Ed Fr 1993; 60 (6bis): 68S–76S.
Fedeli S. Livelli plasmatici di Diacerina nell’huomo anziano dopo somministrazioni repetute del farmaco a posologie diverse. Laboratoires NEGMA-PROTER, Study report, Jul 1988.
Petitjean O, Tod M, Louchahi K, et al. Etude de la pharmacocinétique de l’ART 50® en administration aigüë orale à la dose de 50mg chez le volontaire sain âgé de 61 à 70 ans et de plus de 70 ans, et en administration réitérée à la dose de 50mg × 2/jour chez le volontaire âgé de plus de 70 ans. Laboratoires NEGMA, Study PC/ART 9013N, Study report, Apr 1991.
Vermerie N, Kusielewicz D, Tod M, et al. Pharmacokinetics of glafenine and glafenic acid in patients with cirrhosis, compared to healthy volunteers. Fundam Clin Pharmacol 1992; 6: 197–203.
Orlando R, Fragasso A, Benvenutti C, et al. Pharmacokinetics of rokitamycin after single and repeated dose oral administration to patients with liver cirrhosis. Drug Invest 1991; 3: 162.
Pacifici GM, Viani A, Franchi M, et al. Conjugation pathways in liver disease. Br J Clin Pharmacol 1990; 30: 427–35.
Craig RM, Murphy P, Gibson TP, et al. Kinetic analysis of Dxylose absorption in normal subjects and in patients with chronic renal failure. J Lab Clin Med 1983; 101: 496–506.
Gibson TP, Atkinson AJ, Matusik E, et al. Kinetics of procainamide and N-acetylprocainamide in renal failure. Kidney Int 1977; 12: 422–9.
Gibson TP, Granneman RG, Kallal JE, et al. Cefsulodin kinetics in renal impairment. Clin Pharmacol Ther 1982; 31: 602–8.
Lunell E, Borga O, Larsson R. Pharmacokinetics of enprofylline in patients with impaired renal function. Eur J Clin Pharmacol 1984; 26: 87–93.
Gugler R, Kürten JW, Jensen CJ, et al. Clofibrate disposition in renal failure and acute and chronic liver disease. Eur J Clin Pharmacol 1979; 15: 341–7.
Caldwell J, Hutt AJ, Fournel-Gigleux S. The metabolic chiral inversion and dispositional enantioselectivity of the 2-arylpropionic acids and their biological consequences. Biochem Pharmacol 1988; 37: 105–14.
Barre J, Ledudal P, Tillement JP. Etude in vitro des interactions médicamenteuses sur la fixation plasmatique de la rhéine, métabolite actif de la diacéthylrhéine (diacerein). Laboratoires NEGMA, Study PC/ART 90–39 N, Study report, Dec 1990.
Nguyen M, Dougados M, Berdah L, et al. Diacerein in the treatment of osteoarthritis of the hip. Arthritis Rheum 1994; 37: 529–36.
Hattori M, Namba T, Akao T, et al. Metabolism of sennosides by human intestinal bacteria. Pharmacology 1988; 36 Suppl. 1: 172–9.
Gimminger W, Leng-Peschlow E. Instability of rhein-9-anthrone as a problem in pharmacological and analytical use. Pharmacology 1988; 36 Suppl. 1: 129–37.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicolas, P., Tod, M., Padoin, C. et al. Clinical Pharmacokinetics of Diacerein. Clin Pharmacokinet 35, 347–359 (1998). https://doi.org/10.2165/00003088-199835050-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199835050-00002