Abstract
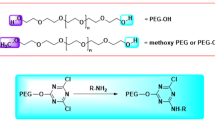
The term PEGylation describes the modification of biological molecules by covalent conjugation with polyethylene glycol (PEG), a non-toxic, non-immunogenic polymer, and is used as a strategy to overcome disadvantages associated with some biopharmaceuticals. PEGylation changes the physical and chemical properties of the biomedical molecule, such as its conformation, electrostatic binding, and hydrophobicity, and results in an improvement in the pharmacokinetic behavior of the drug. In general, PEGylation improves drug solubility and decreases immunogenicity. PEGylation also increases drug stability and the retention time of the conjugates in blood, and reduces proteolysis and renal excretion, thereby allowing a reduced dosing frequency. In order to benefit from these favorable pharmacokinetic consequences, a variety of therapeutic proteins, peptides, and antibody fragments, as well as small molecule drugs, have been PEGylated.
This paper reviews the chemical procedures and the conditions that have been used thus far to achieve PEGylation of biomedical molecules. It also discusses the importance of structure and size of PEGs, as well as the behavior of linear and branched PEGs. A number of properties of the PEG polymer — e.g. mass, number of linking chains, the molecular site of PEG attachment — have been shown to affect the biological activity and bioavailability of the PEGylated product. Releasable PEGs have been designed to slowly release the native protein from the conjugates into the blood, aiming at avoiding any loss of efficacy that may occur with stable covalent PEGylation.
Since the first PEGylated drug was developed in the 1970s, PEGylation of therapeutic proteins has significantly improved the treatment of several chronic diseases, including hepatitis C, leukemia, severe combined immunodeficiency disease, rheumatoid arthritis, and Crohn disease. The most important PEGylated drugs, including pegademase bovine, pegaspargase, pegfilgrastim, interferons, pegvisomant, pegaptanib, certolizumab pegol, and some of the PEGylated products presently in an advanced stage of development, such as PEG-uricase and PEGylated hemoglobin, are reviewed. The adaptations and applications of PEGylation will undoubtedly prove useful for the treatment of many previously difficult-to-treat conditions.










Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Maeda H. SMANCS and polymer conjugated macromolecular drags: advances in cancer therapeutics. Adv Drag Del Rev 1991; 6: 181–202
Torchilin VP. Immobilised enzymes as drugs. Adv Drag Del Rev 1987; 1: 41–86
Abuchowski A, Van E, Palczuk NC, et al. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem 1977; 252: 3578–81
Israelachvili J. The different faces of poly(ethylene glycol). Proc Natl Acad Sci U S A 1997; 94: 8378–9
Harris JM, Chess RB. Effect of PEGylation on pharmaceuticals. Nat Rev Drag Discov 2003; 2: 214–21
Kinstler OB, Brems DN, Lauren SL, et al. Characterization and stability of N-terminally PEGylated rhG-CSF. Pharm Res 1996; 13: 996–1002
Veronese FM, Saccà B, Polverino de Laureto P, et al. New PEGs for peptide and protein modification, suitable for identification of the PEGylation site. Bioconjug Chem 2001; 12: 62–70
Veronese FM. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials 2001; 22: 405–17
Sato H, Ikea M, Suzuki K, et al. Site-specific modification of interleukin-2 by the combined use of genetic engineering techniques and transglutaminase. Biochemistry 1996; 35: 13072–80
Balan S, Choi JW, Godwin A, et al. Site-specific PEGylation of protein disulfide bonds using a three-carbon bridge. Bioconjug Chem 2007; 18: 61–76
Zhao H, Yang K, Martinez A, et al. Linear and branched bicine linkers for releasable PEGylation of macromolecules: controlled release in vivo and in vitro from mono- and multi-PEGylated proteins. Bioconjug Chem 2006; 17: 341–51
Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drag Del Rev 2002; 54: 459–76
Levy Y, Hershfield MS, Fernandez-Mejia C, et al. Adenosine deaminase deficiency with late onset or recurrent infections: response to treatment with poly(ethylene glycol) modified adenosine deaminase. J Pediatr 1988; 113: 312–7
Graham LM. Pegasparaginase: a review of clinical studies. Adv Drag Del Rev 2003; 10: 1293–302
Hak LJ, Relling MV, Cheng C, et al. Asparaginase pharmacodynamics differ by formulation among children with newly diagnosed acute lymphoblastic leukemia. Leukemia 2004; 18: 1072–7
Wang YS, Youngster S, Grace M, et al. Structural and biological characterization of pegylated recombinant interferon alpha-2b and its therapeutic implications. Adv Drag Deliv Rev 2002; 54: 547–70
Monkarsh SP, Ma Y, Aglione A, et al. Positional isomers of mono-pegylated interferon α-2a: isolation, characterization, and biological activity. Anal Biochem 1997; 247: 434–40
Roelfsema F, Biermasz NR, Pereira AM, et al. Nanomedicines in the treatment of acromegaly: focus on pegvisomant. Int J Nanomedicine 2006; 1: 385–98
Pasut G, Veronese FM. Polymer-drug conjugation, recent achievements and general strategies. Prog Polym Sci 2007; 32: 933–61
Blick S, Curran M. Certolizumab pegol. Biodrugs 2007; 21: 196–201
Inada Y, Takahashi K, Yoshimoto T, et al. Application of PEG-enzyme and magnetite-PEG-enzyme conjugates for biotechnological processes. Trends Biotechnol 1988; 6: 131–4
Harris JM. Polyethylene glycol chemistry, biochemical and biochemical applications. New York: Plenum Press, 1992
Harris JM, Zalipsky S, editors. Polyethylene glycol, chemistry and biological applications. Washington, DC: American Chemical Society, 1997
Veronese FM, Harris JM. Peptide and protein PEGylation III: advances in chemistry and clinical applications. Adv Drag Deliv Rev 2008; 60(1): 1–2
Greenwald RB. PEG drags: an overview. J Control Release 2001; 74: 159–71
Pasut G, Guiotto A, Veronese FM. Protein, peptide and non-peptide drug PEGylation for therapeutic application. Exp Opin Ther Pat 2004; 14: 859–94
Banci L, Bertini I, Caliceti P, et al. Spectroscopic characterization of polyethylene glycol Superoxide dismutase: 1H-NMR studies on its Cu22CO2 derivative. J Inorg Biochem 1990; 39: 149–59
Greenwald RB, Choe YH, McGuire J, et al. Effective drag delivery by PEGylated drug conjugates. Adv Drag Deliv Rev 2003; 55: 217–50
Harris JM, Sedaghat-Herati MR. Preparation and use of polyethylene glycol propionaldehyde [US patent 5252714; online]. Available from URL: http://patft.uspto.gov/netahtml/PTO/srchnum.htm [Accessed 2008 Aug 26]
Takakura Y, Fujita T, Hashida M, et al. Disposition of macromolecules in tumor bearing mice. Pharm Res 1990; 7: 339–46
Sartore L, Caliceti P, Schiavon O, et al. Accurate evaluation method of the polymer content in monomethoxy(polyethylene glycol) modified proteins based on amino acid analysis. Applied Biochem Biotechnol 1991; 31: 213–22
Miron T, Wilchek M. A simplified method for the preparation of succinimidyl carbonate polyethylene glycol for coupling to proteins. Bioconjug Chem 1993; 4: 568–9
Veronese FM, Largajolli R, Boccu E, et al. Activation of monomethoxy(poly-ethylene glycol) by phenylchloroformiate and modification of ribonuclease and Superoxide dismutase. Appl Biochem Biotechnol 1985; 11: 141–52
Lee S, McNemar C. Substantially pure histidine-linked protein polymer conjugates [US patent 5985263; online]. Available from URL: http://patft.uspto.gov/netahtml/PTO/srchnum.htm [Accessed 2008 Aug 26]
Morpurgo M, Veronese FM, Kachensky D, et al. Preparation and characterization of polyethylene glycol vinyl sulfone. Bioconjug Chem 1996; 7: 2417–24
Woghiren C, Sharma B, Stein S. Protected thiol-polyethylene glycol: a new activated polymer for reversible protein modification. Bioconjug Chem 1993; 4: 314–8
Veronese FM, Mero A, Caboi F, et al. Site-specific PEGylation of G-CSF by reversible denaturation. Bioconjug Chem 2007; 18: 1824–30
Kopchick JJ, Parkinson C, Stevens EC, et al. Growth hormone receptor antagonist: discovery, development, and use in patients with acromegaly. Endocr Rev 2002; 23: 623–46
Zalipsky S, Meno-Rudolph S. Hydrazide derivatives of polyethylene glycol and their bioconjugates, in polyethylene glycol chemistry and biological applications. In: Harris JM, Zalipsky S, editors. ACS Symposium Series 1997; 680: 318–41
Tayar E, Zhao X, Bentley MD. PEG-LHRH analog conjugates [world patent publication no. WO/1999/055376; online]. Available from URL: http://www.wipo.int/pctdb/en/ [Accessed 2008 Aug 26]
Orsatti L, Veronese FM. An unusual coupling of poly(ethylene glycol) to tyrosine residues in epidermal growth factor. J Bioac Comp Polymer 1999; 14: 429–36
DeFrees S, Wang ZG, Xing R, et al. GlycoPEGylation of recombinant therapeutic proteins produced in Escherichia coll. Glycobiology 2006; 16: 833–43
Gaertner HF, Puigserver AJ. Increased activity and stability of poly(ethylene glycol)-modified trypsin. Enzyme Microb Technol 1992; 14: 150–5
Fontana A, Spolaore B, Mero A, et al. Site-specific modification and PEGylation of pharmaceutical proteins mediated by transglutaminase. Adv Drug Del Rev 2008; 60: 13–28
Messersmith PB, Hu B-H, Ritter Jones M. Injectable and bioadhesive polymeric hydrogels as well as related methods of enzymatic preparation [US patent 7208171; online]. Available from URL: http://patft.uspto.gov/netahtml/PTO/srchnum.htm [Accessed 2008 Aug 26]
Monfardini C, Schiavon O, Caliceti P, et al. A branched monomethoxypoly-(ethylene glycol) for protein modification. Bioconjug Chem 1995; 6: 62–9
Veronese FM, Caliceti P, Schiavon O. Branched and linear poly(ethylene glycol): influence of the polymer structure on enzymological, pharmacokinetic, and immunological properties of protein conjugates. J Bioact Comp Polym 1997; 12: 196–207
Srividhya M, Preethi S, Gnanamani A, et al. Sustained release of protein from polyethylene glycol incorporated amphiphilic comb like polymers. Int J Pharm 2006; 326: 119–27
Bailon P, Palleroni A, Schaffer CA, et al. Rational design of a potent, long-lasting form of interferon: a 40 kDa branched polyethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis C. Bioconjug Chem 2001; 12: 195–202
Delgado C, Francis GE, Fisher D. The uses and properties of PEG-linked proteins. Crit Rev Ther Drug Carrier Syst 1992; 9: 249–304
Caliceti P, Schiavon O, Veronese FM. Immunological properties of uricase conjugated to neutral soluble polymers. Bioconjug Chem 2001; 12: 515–22
Morpurgo M, Veronese FM. Conjugates of peptides and proteins to polyethylene glycols. Methods Mol Biol 2004; 283: 45–70
Federico R, Cona A, Caliceti P, et al. Histaminase PEGylation: preparation and characterization of a new bioconjugate for therapeutic application. J Control Release 2006; 115: 168–74
Visentin R, Pasut G, Veronese FM, et al. Highly efficient technetium-99m labeling procedure based on the conjugation of N-[N-(3-diphenylphosphinopropionyl) glycil] cysteine ligand with poly(ethylene glycol). Bioconjug Chem 2004; 15: 1046–54
Pradhananga S, Wilkinson I, Ross RJ. Pegvisomant: structure and function. J Mol Endocrinol 2002; 29: 11–4
Bell EA, Wall GC. Pediatric constipation therapy using guidelines and polyethylene glycol 3350. Ann Pharmacother 2004; 38: 686–93
Pashankar DS, Loening-Baucke V, Bishop WP. Polyethylene glycol 3350 without electrolytes: a new safe, effective, and palatable bowel preparation for colonoscopy in children. J Pediatr 2004; 144: 358–62
Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of polyethylene glycol with different molecular weights after intravenous administration to mice. J Pharm Sci 1994; 83: 601–6
Kawai F. Microbial degradations of polyethers. Appl Microbiol Biotechnol 2002; 58: 30–8
Beranova M, Wasserbauer R, Vancurova D, et al. Effect of cytochrome P-450 inhibition and stimulation on intensity of polyethylene degradation in microsomal fraction of mouse and rat livers. Biomaterials 1990; 11: 521–4
Working PK, Newman MS, Johnson J, et al. Safety of poly(ethylene glycol) and poly(ethylene glycol) derivatives. In: Harris JM, Zalipsky S, editors. Polyethylene glycol chemistry and biological applications. ACS Symposium Series 1997; 680: 45–57
Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 2006; 1: 297–315
Bendele A, Seely J, Richey C, et al. Short communication: renal tubular vacuolation in animals treated with poly(ethylene glycol)-conjugated proteins. Toxicol Sci 1999; 42: 152–7
Webster R, Didier E, Harris P, et al. Pegylated proteins: evaluation of their safety in the absence of definitive metabolism studies. Drug Metabol Dis 2007; 35: 9–16
Fibbe WE, Daha MR, Hiemstra PS, et al. Interleukin-1 and poly(rI)-poly(rC) induce production of granulocyte CSF, macrophage CSF and granulocyte-macrophage CSF by human endothelial cells. Exp Hematol 1989; 17: 229–34
Weite K, Platzer E, Lu L, et al. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A 1985; 82: 1526–30
Lu HS, Clogston CL, Narhi LO, et al. Folding and oxidation of recombinant human granulocyte colony stimulating factor produced in Escherichia coli: characterization of the disulfide-reduced intermediates and cysteine-serine analogs. J Biol Chem 1992; 267: 8770–7
Kinstler O, Molineaux G, Treuheit M, et al. Mono-N-terminal poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev 2002; 54: 477–86
Molineaux G. The design and development of pegfilgrastim (PEG-rmetHuG-CSF, Neulasta). Curr Pharm Des 2004; 10: 1235–44
Piedmonte DM, Treuheit MJ. Formulation of Neulasta® (pegfilgrastim). Adv Drug Del Rev 2008; 60: 50–8
Luxon BA, Grace M, Brassard D, et al. Pegylated interferons for the treatment of chronic hepatitis C infection. Clin Ther 2002; 24: 1363–83
Foster GR. Pegylated interferons: chemical and clinical differences. Aliment Pharmacol Ther 2004; 20: 825–30
Hinds KD, Kim SW. Effect of PEG conjugation on insulin properties. Adv Drug Deliv Rev 2002; 54: 505–30
Hershfield MS, Chaffee S, Koro-Johnson L, et al. Use of site-directed mutagenesis to enhance the epitope-shielding effect of covalent modification of proteins with polyethylene glycol. Pro Natl Acad Sci U S A 1991; 88: 7185–9
Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci Epub 2008 Jan 15
Parkinson C, Scarlett JA, Trainer PJ. Pegvisomant in the treatment of acromegaly. Adv Drug Deliv Rev 2003; 55: 1303–14
Ng EWM, Shima DT, Calias P, et al. A targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Dis 2006; 5: 123–32
Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Del Rev 2002; 54: 531–45
Chapman AP, Antoniw P, Spitali M, et al. Therapeutic antibody fragments with prolonged in vivo half-lives. Nat Biotechnol 1999; 17: 780–3
Melmed GY, Targan SR, Yasothan U, et al. Certolizumab pegol. Nat Rev Drug Discov 2008; 7: 641–2
Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. PRECISE 1 Study Investigators. N Engl J Med 2007; 357: 228–38
Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. PRECISE 2 Study Investigators. N Engl J Med 2007; 357: 239–50
Fleischmann R, Mason D, Cohen S. Efficacy and safety of certolizumab pegol monotherapy in patients with rheumatoid arthritis failing previous DMARD therapy [abstract]. Ann Rheum Dis 2007; 66Suppl. II: 169
Smolen J, Brzezicki J, Mason D, et al. Efficacy and safety of certolizumab pegol in combination with methotrexate (MTX) in patients with active rheumatoid arthritis despite MTX therapy: results from the RAPID 2 study [abstract]. Ann Rheum Dis 2007; 66Suppl. II: 187
Reich K, Tasset C, Ortonne J. Efficacy and safety of certolizumab pegol in patients with chronic plaque psoriasis: preliminary results of a randomised, double-blind, placebo-controlled trial [abstract]. Ann Rheum Dis 2007; 66Suppl. II: 251
Edwards C. PEGylated recombinant human soluble tumour necrosis factor receptor type I (r-Hu-sTNF-RI): novel high affinity TNF receptor designed for chronic inflammatory diseases. Ann Rheum Dis 1999; 58: 173–81
Paz K, Zhu Z. Development of angiogenesis inhibitors to vascular endothelial growth factor receptor 2: current status and future perspective. Front Biosci 2005; 10: 1415–39
Nishimura H, Ashihara Y, Matsushima A, et al. Modification of yeast uricase with poly(ethylene glycol): disappearance of binding ability towards anti-uricase serum. Enzyme 1979; 24: 261–4
Abuchowski A, Karp D, Davis FF. Reduction of plasma urate levels in the cockerel with poly(ethylene glycol)-uricase. J Pharmacol Exp Ther 1981; 219: 352–4
Caliceti P, Morpurgo M, Schiavon O, et al. Preservation of thrombolytic activity of urokinase modified with monomethoxypoly(ethylene glycol). J Bioact Comp Polym 1999; 4: 252–66
Chua CC, Greenberg ML, Viau AT, et al. Use of poly(ethylene glycol)-modified uricase (PEG-uricase) to treat hyperuricemia in a patient with non-Hodgkin lymphoma. Ann Intern Med 1988; 109: 114–7
Coiffier B, Mounier N, Bologna S, et al. Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-Hodgkin’s lymphoma: results of the GRAAL1 (Groupe d’Etude des Lymphomes de l’Adulte Trial on Rasburicase Activity in Adult Lymphoma) study. J Clin Oncol 2003; 21: 4402–6
Sherman MR, Saifer MGP, Perez-Ruiz F. PEG-uricase in the management of treatment-resistant gout and hyperuricemia. Adv Drug Del Rev 2008; 60: 59–68
Sundy JS, Ganson NJ, Kelly SJ, et al. Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum 2007; 56: 1021–8
Ganson NJ, Kelly SJ, Scarlett E, et al. Control of hyperuricemia in subjects with refractory gout, and induction of antibody poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther 2006; 8(1): R12
Richter AW, Akerblom E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int Arch Allergy Appl Immunol 1983; 74: 124–31
Fisher TC, Armstrong JK, Wenby RW, et al. Isolation and identification of a human antibody to poly(ethylene glycol) [abstract]. Blood 2003; 102: 559
Hess JR, Macdonald VM, Brinkley WW. Systemic and pulmonary hypertension after resuscitation with cell free hemoglobin. J Appl Physiol 1993; 74: 1769–78
Nho K, Zalipsky S, Davis F. Chemically modified hemoglobin as an effective, stable, non-immunogenic red blood cell substitute [US patent 5386014; online]. Available from URL: http://patft.uspto.gov/netahtml/PTO/srchnum.htm [Accessed 2008 Aug 26]
Manjula BN, Tsai A, Upadhya R, et al. Site-specific PEGylation of hemoglobin at Cys-93β: correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjug Chem 2003; 14: 464–72
Hu T, Prabhakaran M, Acharya S, et al. Influence of the chemistry of conjugation of polyethylene glycol to Hb on the oxygen-binding and solution properties of the PEG-Hb conjugate. Biochem J 2005; 392: 555–64
Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov 2003; 2: 347–60
Zhao H, Rubio B, Sapra P, et al. Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjug Chem 2008; 19: 849–59
Acknowledgments
The authors thank UCB for providing editorial support for the preparation of this paper, and Dr G. Pasut for carefully reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veronese, F.M., Mero, A. The Impact of PEGylation on Biological Therapies. BioDrugs 22, 315–329 (2008). https://doi.org/10.2165/00063030-200822050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200822050-00004