Abstract
Spray-dried monoclonal antibody (mAb) powders may offer applications more versatile than the freeze-dried cake, including preparing high-concentration formulations for subcutaneous administration. Published studies on this topic, however, are generally scarce. This study evaluates a pilot-scale spray dryer against a laboratory-scale dryer to spray-dry multiple mAbs in consideration of scale-up, impact on mAb stability, and feasibility of a high-concentration preparation. Under similar conditions, both dryers produced powders of similar properties—for example, water content, particle size and morphology, and mAb stability profile—despite a 4-fold faster output by the pilot-scale unit. All formulations containing arginine salt or a combination of arginine salt and trehalose were able to be spray-dried with high powder collection efficiency (>95%), but yield was adversely affected in formulations with high trehalose content due to powder sticking to the drying chamber. Spray-drying production output was dictated by the size of the dryer operated at an optimal liquid feed rate. Spray-dried powders could be reconstituted to high-viscosity liquids, >300 cP, substantially beyond what an ultrafiltration process can achieve. The molar ratio of trehalose to mAb needed to be reduced to 50:1 in consideration of isotonicity of the formulation with mAb concentration at 250 mg/mL. Even with this low level of sugar protection, long-term stability of spray-dried formulations remained superior to their liquid counterparts based on size variant and potency data. This study offers a commercially viable spray-drying process for biological bulk storage and an option for high-concentration mAb manufacturing.
LAY ABSTRACT: This study evaluates a pilot-scale spray dryer against a laboratory-scale dryer to spray-dry multiple monoclonal antibodies (mAbs) from the perspective of scale-up, impact on mAb stability, and feasibility of a high-concentration preparation. The data demonstrated that there is no process limitation in solution viscosity when high-concentration mAb formulations are prepared from spray-dried powder reconstitution compared with concentration via the conventional ultrafiltration process. This study offers a commercially viable spray-drying process for biological bulk storage and a high-concentration mAb manufacturing option for subcutaneous administration. The outcomes of this study will benefit scientists and engineers who develop high-concentration mAb products by providing a viable manufacturing alternative.
- Spray drying
- Monoclonal antibody (mAb)
- Powder collection yield
- Powder reconstitution
- Water content
- Scale-up
Introduction
We previously reported that a laboratory-scale, high-efficiency spray dryer enables spray-drying multiple commercial monoclonal antibodies (mAbs) with >95% powder collection efficiency, and we demonstrated that mAbs are amenable to the transient, high-temperature stress (∼200 °C) during spray drying (1). Despite these promising data, it is still premature to claim that protein spray-drying technology is a viable manufacturing process because the optimal liquid throughput for the laboratory-scale dryer remains low (10–15 mL/min, or 0.6–0.9 L/h). Therefore, the scalability question needs to be addressed and understood. “Manufacturing-scale” spray drying for mAb production is different from what is defined in the chemical or food industry (tons per hour or per day). In biotechnology, a standard 12,000 L fermentation process can produce the final bulk drug substance (DS) (e.g., at 100 mg/mL) in hundreds of liters. Using 200 L as an example, it will take 220–330 h for laboratory-scale equipment to spray-dry the whole batch. Thus, a spray dryer with a 5-fold increase in drying capacity can process 200 L of mAbs within 3 d, which seems to be a reasonable starting point for scale-up consideration. A pilot-scale spray dryer in the existing product line has a maximum drying air rate of 154 kg/h, which is 4.4 times higher than that of the laboratory-scale dryer.
Thus, the objective of this study was twofold. Firstly, the performance of the pilot-scale dryer in spray drying multiple mAbs was evaluated against the laboratory-scale unit in terms of production output, powder collection efficiency, and powder properties such as water content, particle distribution, and particle morphology. The impact of spray drying on short-term and long-term mAb stability was assessed by protein size variant quantitation and potency assay. The second objective was to assess the viability of manufacturing high-concentration/high-viscosity mAb formulations by reconstituting spray-dried powders. Normally, final protein/mAb bulk DS is prepared at the end of the purification process using ultrafiltration/diafiltration (UF/DF). UF/DF may encounter significant challenges with high-viscosity bulk, such as prolonged processing time, decreased recovery, and possible adverse changes in product quality attributes.
Formulation is known to play a critical role in spray drying performance and mAb stability (1⇓⇓–4). Carbohydrate sugars, such as trehalose and sucrose, have demonstrated their effectiveness in stabilizing mAb in the dehydrated state via spray drying (5⇓⇓–8) and lyophilization (9⇓⇓–12), and the 300:1 molar ratio of trehalose to mAb (or proteins)—a 0.7:1 weight ratio—is typically considered sufficient to protect the proteins. Unfortunately, at this level, powder collection efficiency is diminished because of the tackiness of trehalose, and its amount should be decreased to a 0.5:1 weight ratio (220:1 molar ratio) in order to maintain powder collection efficiency at >95% (1). Although a 220:1 molar ratio is still effective in protecting mAbs, other restrictions may require the amount of trehalose to be further reduced: The isotonicity of the liquid formulation for subcutaneous injection and the need to reduce the viscosity of high-concentration mAb liquid formulations must be considered. Thus, the formulations investigated herein contained trehalose, an organic salt, or a combination of both. The inclusion of an organic salt, for example, arginine succinate, was to reduce the viscosity of the high-concentration mAb liquid upon powder reconstitution. The amount of arginine succinate and trehalose, however, needs to be confined in consideration of the formulation's tonicity. Overall, this study reported a scaled-up spray-drying process with mAb formulations tailored to a high-concentration/high-viscosity drug product (DP) manufacturing process.
Materials and Methods
Two recombinant humanized mAbs of the IgG1 subclass, mAb A and mAb B, were manufactured by Genentech (South San Francisco, CA). These mAbs were expressed in Chinese hamster ovary (CHO) cell lines. All mAbs were prepared into comparable levels of mAb concentrations using a tangential-flow filtration unit (Pellicon3 10kD, Millipore, Billerica, MA) and formulated with arginine succinate with or without trehalose dihydrate (Table I). All bulks were buffered to a pH of ∼6.0.
Spray-Drying Formulations of mAb A and mAb B
Spray Drying
Two types of spray dryers were used in this study, a laboratory-scale unit (Anhydro MicraSpray35, SPX Flow Technology Systems, Inc., Elkridge, MD) (MS-35; Figure 1a) and a pilot/manufacturing-scale unit (Anhydro MicraSpray150, also from SPX) (MS-150; Figure 1b). The capacity of the MS-150 dryer is approximately 4 times larger than that of the MS-35 dryer (154 vs 38 kg/h drying air rate). The laboratory-scale unit was constructed mostly of stainless steel with surface heating and insulation (drying chamber, cyclone, etc.) plus a glass vision panel, while the pilot/manufacturing-scale unit was made of stainless steel without surface heating.
Spray dryer designs: (a) MS-35, (b) MS-150 with dual cyclone design, (c) original MS-150 cyclone, (d) design of dual cyclone for MS-150.
Particle Size Analysis
Particle size distribution was measured using a laser diffraction analyzer (LA-950, HORIBA, Ltd., Kyoto, Japan). Based on light scattering, the particle size distribution of a sample can be analyzed and calculated using the Mie theory. For analysis, several milligrams of the spray-dried powders were dispersed in 50 mL of isopropyl alcohol in the instrument's sample handler and sonicated for ∼1 min to disperse the particles prior to analysis.
Water Content
Water content in the spray-dried samples was determined using a volumetric Karl Fischer titration analyzer (DL31, Mettler-Toledo, Columbus, OH). Approximately 100 mg of each sample were injected into the titration cell that contained anhydrous methanol. Hydranal®-Composite 2 volumetric reagent (Riedel-de Haën, Heidelberg, Germany) was used as a titrant.
Size Exclusion Chromatography (SEC)
The quantitation of size variants was determined by SEC. The analysis utilized a G3000SWXL column, 7.8 mm inner diameter (ID) × 30 cm, 5 μm (TOSOH BioScience, King of Prussia, PA) run on a high-performance liquid chromatography (HPLC) system (model 1200, Agilent Technologies, Santa Clara, CA). The mobile phases were 0.2 M potassium phosphate and 0.25 M potassium chloride at pH 6.2 for mAb A and 0.1 M potassium phosphate at pH 6.8 for mAb B. The chromatography was run isocratically at a flow rate of 0.5 mL/min for 30 min. The column temperature was maintained at ambient for both mAb A and mAb B, and the eluent absorbance was monitored at 280 nm. Each mAb was diluted with its respective formulation buffer to 25 mg/mL for mAb A and 10 mg/mL for mAb B. The injection volumes were 10 μL for mAb A and 20 μL for mAb B.
Osmolality Measurement
Osmolality was determined by the freezing-point depression method using an Advanced® 2020-BIO Multi-Sample Osmometer (Advanced Instrument, Inc., Norwood, MA). Each sample (20 μL) was transferred into disposable tubes and loaded onto the osmometer. The osmolality of each sample was determined based on the freezing point of the solution. For the samples that did not freeze, Vapro Osmometer 5520 (Advanced Instrument, Inc.) was used. Each sample (10 μL) was transferred onto a solute-free paper disc in the sample holder. The osmolality of each solution was determined based on the dew-temperature depression.
Viscosity Measurement
Viscosity was measured using a Physica MCR501 controlled stress rheometer (Anton Paar, Ashland, VA) equipped with Rheoplus software. A 75 cm ID cone plate was used for all the analyses. The sample (70 μL) was transferred on the cone plate and subjected to constant shear of 1000/s at 25 °C for 10 s. Ten readings were taken, and an average viscosity was determined.
In Vitro Potency Assay
An in vitro potency assay was used to determine the activity of mAb A by measuring its ability to inhibit vascular endothelial growth factor–induced human umbilical vein endothelial cell (HUVEC) proliferation (13). For determining the activity of mAb B, the method is based on measuring its ability to inhibit proliferation of BT-474 cells using alamarBlue® (Life Technologies Corporation, Grand Island, NY) (14). Samples were reconstituted to 100 mg/mL and diluted with formulation buffer to 0.5 mg/mL before submission for analysis.
Reconstitution Time
Each of the spray-dried powders was reconstituted with 1 mL of purified water to make a protein solution of 25 mg/mL in a 2 mL glass vial. The vial was agitated at 450 rpm on a shaker (model 120, Glas-Col, Terre Haute, IN) at ambient temperature. The time required to completely dissolve the powder was recorded.
Turbidity of Reconstituted Solution
Opalescence of the reconstituted solutions at a protein concentration of 25 mg/mL was measured in a 1 cm path-length cuvette using a UV spectrophotometer (model 8453, Agilent Technologies). The test samples were blanked against purified water. Absorbance was recorded at 340, 345, 350, 355, and 360 nm, and the average of the absorbance readings was reported as the turbidity of the samples.
Results and Discussion
High-Concentration/High-Viscosity DP Process—UF/DF versus Spray Drying
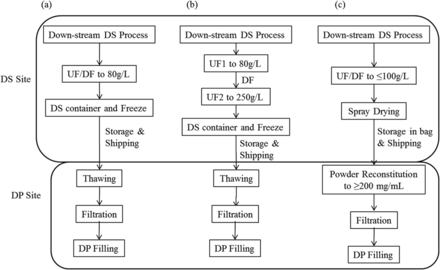
A typical biologics production process features DS and DP manufactured at different sites. The DS site produces the final bulk DS through a series of upstream/downstream unit operations concluded by UF/DF. The bulk is then filled into DS storage containers and is typically frozen for storage and shipping to a DP site where bulk DS can be further compounded (e.g., pooling, dilution, reformulation, etc.) prior to being filled into a primary container/device, (e.g., vials or syringes; Figure 2a). To produce high-concentration mAb bulk DS, (e.g., 200 mg/mL or higher), the conventional single UF/DF process may not be appropriate and can be replaced by a two-stage UF1/DF/UF2 (15) operation as shown in Figure 2b. However, at such a high concentration, gelation can occur at the filter membrane surface and dramatically reduce the permeate flux. Normally, to overcome this challenge, the UF/DF processing temperature is elevated (>25 °C) to reduce viscosity. Unfortunately, this high temperature can raise stability concerns, especially for sensitive proteins. Low DS bulk recovery (i.e., yield) at high viscosity remains a drawback.
Process flow for producing high-concentration mAb formulations: (a) conventional UF/DF process, (b) UF1/DF/UF2 process, (c) reconstitution of spray-dried powder.
An alternative approach is to replace the UF2 process with spray drying of the DS bulk derived from UF/DF (Figure 2c). In this single UF/DF process, the protein concentration can be increased to a reasonable level (e.g., ∼100 mg/mL) to reduce the bulk volume for spray drying. The resulting powder can be stored in a disposable bag. The storage and shipping of dry powder bulk can offer advantages over transporting DS bulk in the liquid or frozen state. At the DP site, the powder can be reconstituted with a diluent into a final high-concentration DS bulk. Our study was based on this concept and focused on developing a scaled-up spray-drying process on multiple mAb formulations tailored to satisfy long-term mAb stability and reconstitution properties.
Formulation Considerations
Formulation strategies are set based on the application of spray-dried powders. In a previous study intended for the application of biologic bulk storage, formulations containing only different levels of trehalose were assessed to evaluate the impact on powder collection efficiency and mAb stabilization during and after spray drying (1). For the current application in powder reconstitution into a high-concentration mAb liquid (≥200 mg/mL) for subcutaneous administration—for example, via a pre-filled syringe—two additional constraints were given to formulation consideration: viscosity reduction at high mAb concentration and maintaining acceptable formulation osmolality.
A potential challenge in the development of high-protein concentration formulations is concentration-dependent solution viscosity (16⇓⇓⇓–20). For subcutaneous injection using a pre-filled syringe, injection force (or glide force) is a complex factor influenced by solution viscosity and the size (gauge) of the needle (21). Smaller needles (e.g., ≥26 gauge) will pose less pain sensation to the patient but require more force to inject a high-viscosity drug. Based on a viscosity–glide force relationship calculated by the Hagen-Poiseuille equation as a function of needle gauge, with a 27 gauge thin-walled needle (ID, minimum: 0.241 mm), the liquid viscosity should be maintained below 20 cP in order not to exceed a glide force of 20 N (21). Unfortunately, formulation scientists are constantly challenged by the conflicting reality of a high mAb concentration and high solution viscosity (14⇓⇓⇓–18). Different formulation strategies have been proposed to reduce the viscosity of liquid solutions at high mAb concentrations, including formulating with organic and inorganic salts to balance repulsive and attractive forces through intermediate ionic strengths (16⇓⇓–19, 22, 23). Of these, arginine salt has been proven effective (17, 18, 20). The impact of arginine salt on spray drying is, however, unproven and was thus investigated in this study. In addition, the concentration of the excipients should be confined to meet the isotonicity requirement of ∼300 mOsmol/kg (24). However, maintaining this level of osmolality for subcutaneous administration remains debatable and may not be necessary (17). A recent randomized, placebo-controlled, double-blind, cross-over trial assessed the effect of osmolality (300, 600, 850, and 1100 mOsmol/kg) administered intramuscularly on local tolerance. The results did not show any dose–effect relationship between burning and pain sensations and the different osmolalities tested (24, 25, 26). As an osmotic effect can be influenced by the volume of injection, which is normally the case for high-dose mAb formulations, our formulation strategy was still to try to keep within a threshold, for example, 600 mOsmol/kg.
With these considerations, all formulations tested for spray drying, as listed in Table I, included four major compounds: mAb, a buffer, trehalose, and/or arginine succinate. Formulations containing arginine salt (A-4, A-5, A-6, B-4, B-5, and B-6) were the focus of this study. The osmolality of each formulation was measured; all formulations prior to spray drying were hypotonic to isotonic (i.e., ≤300 mOsmol/kg) with the exception of A3, B3, and C3, which contained the highest amount of trehalose (>200 mg/mL) and heightened osmolality to >700 mOsmol/kg. When the spray-dried powders of the arginine-containing formulations were reconstituted with water to a mAb concentration of 200 mg/mL, their osmolality would stay below 800 mOsmol/kg.
The laboratory-scale spray dryer MS-35 was equipped with a high-efficiency cyclone, to which the enhanced powder collection efficiency was attributed. The pilot-scale unit MS-150 originally came with a single, larger cyclone (Figure 1c), which was replaced and tested with a dual cyclone unit (Figure 1d). Each of the two cyclones was fabricated according to the cyclone design associated with MS-75 (SPX Flow Technology Systems, Inc., Elkridge, MD; detailed drawing not shown). Two cyclones were coupled to accommodate a 2-fold increase in drying air flow rate. The batch size of each run was also increased per the size of the spray dryer, from 0.10–0.25 L (MS-35) to 0.5–1.0 L (MS-150). To calculate the yield of powder collection, only the powder collected in the receiver was considered. The default drying air inlet and outlet temperatures, Ti at 180–190 °C and To at 80–90 °C, respectively, were applied to both MS-35 and MS-150. Spray drying conditions and the characteristics of the dry powders produced using each spray dryer are listed in Table II.
Specification Comparison and Residence Time Calculation of MS-35 versus MS-150 Spray Dryers
Spray Dryer Scale-Up: Laboratory-Scale (MS-35) versus Pilot-Scale (MS-150)
MS-35 was previously assessed and optimized for spray-drying mAb formulations (1). In that study, high powder-collection efficiency (>95% yield) was reported for three mAb models. Trehalose was effective in mAb stabilization, but powder yield deteriorated with increasing trehalose concentration. In general, the 2:1 weight ratio of mAb:trehalose (i.e., 1:220 molar ratio) offered acceptable stability and good powder yield. A standard drying condition, Ti of 180–190 °C and To of 80–90 °C could produce powders of approximately 5% moisture content when the liquid was dried at a rate of 15 mL/min (i.e., <1 L/h). To increase production scale (output), a pilot-scale spray dryer, MS-150, which enhances drying capacity 4-fold based on drying air flow rate, was assessed in the study presented here. The comparison of MS-35 and MS-150 in drying chamber dimensions and calculated air residence time is summarized in Table II. The data indicate that it takes almost 4 times longer for the drying air to pass through the MS-150 than it does to pass through the MS-35.
All formulations (Table I) were spray-dried using MS-35 and MS-150, and the drying conditions and powder/process characterization results (yield, particle size, and water content) are summarized in Table III. Particle size (median value, D50) was slightly larger when powders were spray-dried using MS-150 (∼15 μm) than when using MS-35 (∼12 μm), but size was not affected by drying conditions or formulation. The water content of all spray-dried powders was mostly around 5% and showed no obvious correlations with formulation, dryer size, or drying conditions. Powder yield was almost fully recovered (>95% yield) for all arginine-containing formulations (A-4, A-5, A-6, B-4, B-5, and B-6) and was very good (>90% yield) for formulations with low levels of trehalose (≥2:1 mAb:trehalose w/w, i.e., A-2 and B-2) for both MS-35 and MS-150. However, yield appears to be influenced by several factors:
Formulation. It is consistent with the previous finding (1) that formulations with high trehalose concentrations (for example, 1:2 mAb:trehalose w/w for A-3 and B-3) are difficult to recover because a significant amount of the powder sticks to the drying chamber.
Cyclone design. The dual-cyclone system (Figure 1d) outperforms the original single cyclone system (Figure 1c). Each cyclone of the dual system has a design concept similar to that of the MS-35 as described previously (1). The dual cyclone was designed for the collection of fine biopharmaceutical powders and has a cut-off size of 1.5 μm, as calculated using a developed mathematical model (27). The original single-cyclone design failed to effectively collect small particles because it is designed for general cyclone application and has a cut-off size of 6 μm. This resulted in <50% collection efficiency for A-2* and B-2* (Table III) compared with >90% yield for A-2 and B-2, which were collected using the dual cyclone system.
Drying conditions. The liquid feed rate is a critical parameter. Powder yield decreased when the liquid feed rate was increased from 50 mL/min for A-2 (31% at 102 mL/min and 45% at 84 mL/min). Yield loss occurred primarily in the drying chamber.
Spray-Drying Conditions of MS-150 and MS-35 and Summary of Results
The scalability of the spray-drying process is obvious between MS-35 and MS-150 based on the rate of drying air and the rate of water evaporation (liquid feed rate). Under the similar drying conditions, both dryers produced powders of similar water content, particle size, and powder yield (if the similar high-efficiency cyclone was used). The 4-fold increase in drying rate, that is, the liquid feed rate of 50 mL/min (MS-150) versus 15 mL/min (MS-35), reflected the 4-fold increase in the drying air rate, 154 kg/h versus 38 kg/h. The attempt to further enhance the production rate by increasing the liquid feed rate failed, suggesting that the current drying conditions were optimized and further scale-up can only rely on using an even larger spray dryer.
MAb Stability/Potency of Spray-Dried Powder Formulation
It was previously reported (1) that, despite a high inlet temperature (∼190 °C), the physical stability (determined by SEC-HPLC) of spray-dried mAbs was comparable to or greater than that of freeze-dried counterparts. In addition, a carbohydrate sugar (e.g., trehalose) was essential in stabilizing mAbs during spray drying and long-term storage, particularly under the stressed condition (40 °C). The stabilization benefit increased with increasing amounts of trehalose, but the lowest amount of trehalose tested in that study was a 1:220 molar ratio of mAb:trehalose. Stability of the mAb was only assessed based on the protein sizing assay (SEC-HPLC).
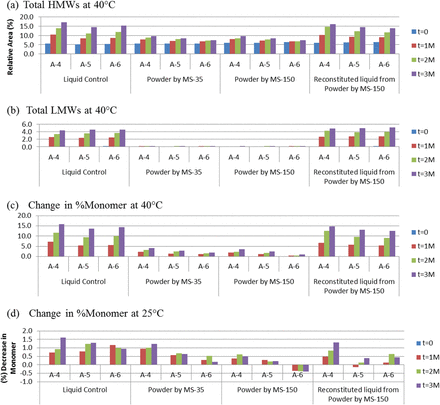
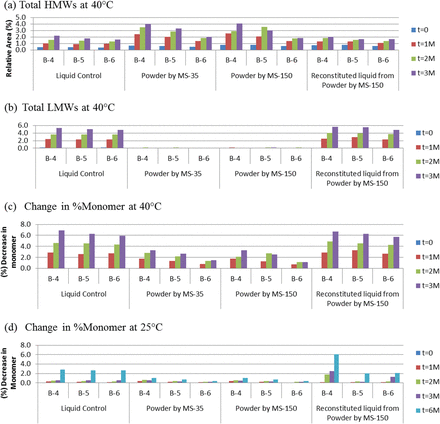
In the current study, the amount of trehalose was further reduced to decrease osmolality in the arginine-containing formulations, which contained either no trehalose (A-4 and B-4) or one of two mAb:trehalose molar ratios, 1:38 (A-5 and B-5) and 1:150 (A-6 and B-6). All three formulations were spray-dried using the MS-35 and MS-150 units, and the powder samples were stored in vials for up to 3 months at 40 °C and up to 6 months at 25 °C. Two liquid controls, liquid formulations prior to spray drying and reconstituted (into 100 mg/mL) from spray-dried powders, were stored under the same conditions to compare with powder samples. In addition to SEC-HPLC analysis (quantifying size variants), the biological potency of mAbs and turbidity of the solution were also determined. Figure 3 summarizes the size variant (SEC-HPLC) data for mAb A formulations for 3 months at 40 °C and 25 °C, while the size variant data for mAb B are presented in Figure 4 for 3 months at 40 °C and 6 months at 25 °C. The size variant data include high-molecular-weight species (HMWs) due to aggregation, low-molecular-weight species (LMWs) due to fragmentation, and total percent monomer (%monomer).
Effect of spray drying and effect of long-term storage of liquid and spray-dried formulations (A4–A6 for mAb A) on (a) HMWs before and after 3 months at 40 °C, (b) LMWs before and after 3 months at 40 °C, (c) total %monomer before and after 3 months at 40 °C, (d) total %monomer before and after 3 months at 25 °C.
Effect of spray drying and effect of long-term storage of liquid and spray-dried formulations (B4–B6 for mAb B) on (a) HMWs before and after 3 months at 40 °C, (b) LMWs before and after 3 months at 40 °C, (c) total %monomer before and after 3 months at 40 °C, (d) total %monomer before and after 6 months at 25 °C.
High-Molecular-Weight Species (HMWs):
Both antibodies aggregated slightly, <1% increase in HMWs, right after spray drying at 190 °C, even for formulations containing no trehalose (A-4 and B-4; Figures 3a and 4a, respectively) although the starting level of HMWs was different between mAb A (>5%) and mAb B (<0.5%). The differences between the two spray dryers were minimal. After long-term storage, mAb aggregation in powder formulations increased with enhanced storage temperature and decreasing trehalose concentration. When powder formulations were compared with the liquid formulations prior to spray drying, mAb A and mAb B behaved differently. The liquid formulations of mAb A aggregated faster than the powder counterparts, while the liquid formulations of mAb B were more stable than the corresponding powders. The stabilization effect of trehalose was more prominent in the solid state than in the liquid state. When liquid formulations reconstituted from the spray-dried powders were compared with liquid formulations prior to spray drying, their tendency to aggregate or fragment was similar, suggesting that the effect of high-temperature spray drying on long-term storage is benign.
Low-Molecular-Weight Species (LMWs) (Fragmentation in Figures 3b and 4b):
Fragmentation occurred minimally in the solid state, while it was a major degradation pathway in the liquid state for both mAb A and mAb B (Figures 3b and 4b, respectively). The ability of trehalose to protect mAb from fragmenting played an insignificant role.
Change (Decrease) in %Monomer:
For total decrease in %monomer (relative to liquid formulation prior to spray drying), similar trends were observed: powder formulations being more stable than liquid counterparts; increasing trehalose concentration resulting in less degradation; stability profile of liquid formulations prior to spray drying and reconstituted from spray-dried powders being identical; powders spray dried from MS-35 and MS-150 showing no significant differences (Figures 3c, 3d, 4c, and 4d, respectively). Overall, trehalose effectively stabilized mAbs at mAb:trehalose molar ratios as low as 1:38.
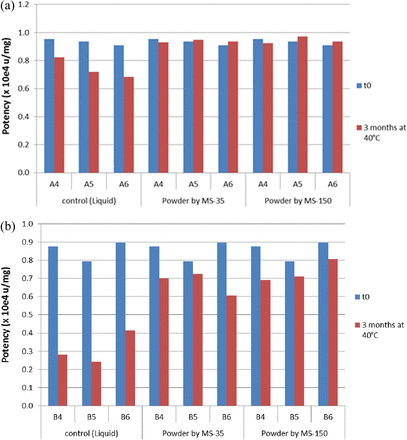
In Vitro Potency Stability
The in vitro potency assay for mAb A and mAb B was based on the ability of the two mAbs to inhibit the proliferation of HUVEC cells (13) and BT-474 cells (14), respectively. All liquid and dry powder formulations at t = 0 and t = 3 months at 40 °C were analyzed, and the results are presented for mAb A and mAb B in Figure 5a and Figure 5b, respectively. The activity of all mAb formulations after spray drying was comparable to that of the respective liquid formulations prior to spray drying, suggesting the impact of high-temperature spray drying is insignificant. Incubation of liquid formulation for 3 months at 40 °C substantially reduced their activity by 20–30% for mAb A and 50–70 % for mAb B. Incubation of powder formulations for 3 months at 40 °C has little impact on the potency of mAb A and approximately 30% potency decrease for mAb B. Although aggregation is the main degradation pathway for dry powder formulations, it is possible that aggregated mAb species remain active. The LMWs might be less potent or inactive, as fragmentation is the major degradation mechanism for liquid formulations. Although trehalose protects mAb from degradation (aggregation), it shows no obvious effect in retaining mAb potency, either in the liquid state or the solid state.
Effect of spray drying and effect of long-term storage of liquid and spray-dried formulations on in vitro potency of (a) mAb A formulation (A4–A6) and (b) mAb B formulation (B4–B6).
Solution Turbidity
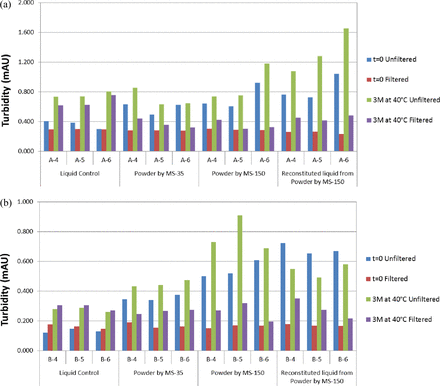
Solution turbidity may be an implication of protein instability (i.e., the presence of insoluble aggregated protein particles). Turbidity is also important from the process perspective because the spray-dried powder will be reconstituted into a high-concentration mAb liquid prior to bioburden reduction and sterile filtration. A turbid solution may tend to clog the filter pores and foul the membrane to slow down or interrupt the manufacturing process. All liquid formulations, including those prior to spray drying and reconstituted solutions from spray-dried powders before and after 3 month incubation at 40 °C, were UV-scanned before and after filtration through a 0.22 μm filter. The turbidity results for mAb A and mAb B are summarized in Figures 6a and 6b, respectively.
Effect of spray drying and effect of long-term storage of liquid and spray-dried formulations on solution turbidity before and after 40 °C storage, and before and after 0.22 μm sterile filtration for (a) mAb A (A4–A6) and (b) mAb B (B4–B6).
Solutions of reconstituted spray-dried powders are generally more turbid than the respective liquid formulations prior to spray drying, suggesting the presence of insoluble protein aggregates. The amount of these aggregates, however, is difficult to quantify and may be insignificant, as protein concentration remains the same after filtration. Solution turbidity appeared to increase slightly after 3 month incubation at 40 °C for both powder and liquid formulations, but there was no obvious trend among the different formulations. These particles were removable upon sterile filtration (0.22 μm); after filtration, solution turbidity was comparable to the unfiltered liquids even after 3 month storage under a stressed condition. The impact of solution turbidity on filterability (i.e., filter fouling) is out of the scope of this study and will be evaluated separately.
Table IV summarizes the time required to reconstitute the spray-dried powders (A4–A6 and B4–B6) into 25 mg/mL concentration. All powders were completely dissolved within 3 min. It took slightly longer time to dissolve powders produced from the pilot-scale dryer, 2–3 min, than the lab-scale dryer, 1–2 min, probably because of the larger particle size of the powders from MS-150. Reconstitution into higher concentrations will certainly take much longer time. However, reconstitution will take place at the DP manufacturing site using qualified mixing equipment and pre-characterized mixing conditions, which is beyond the scope of this study.
Time Required for Reconstituting Spray-Dried Powders into 25 mg/mL
Reconstitution into High-Concentration/High-Viscosity Formulations
Spray-dried powders were reconstituted into liquids with mAb concentrations of 100, 150, and 250 mg/mL. Their viscosity and osmolality were measured and are summarized in Table V. Compared with liquid formulations prior to spray drying, reconstituted liquids at the same concentration (100 mg/mL) had comparable viscosity (∼3–4 cP) and osmolality (200–300 mOsmol/kg). To further increase mAb concentration via UF/DF, the associated increase in viscosity may limit the capability of the standard UF/DF process. Internal manufacturing data suggested that UF remains a feasible method to concentrate mAbs to the viscosity range of 15 to 30 cP but with reduced process yield. UF is not capable of producing mAb formulations beyond 30 cP, which requires substantially longer processing time and results in lower yields. Although performing UF/DF at a higher temperature (e.g., 30 or 40 °C) can decrease viscosity to enable the process, it may risk compromising mAb quality. The reconstitution approach has no such limitations; A-6 formulation viscosity reached beyond 200 cP and beyond 300 cP for B-6 at 250 mg/mL mAb concentration. Comparing formulations containing trehalose (A-4, B-4, A-5, and B-5) with other formulations, it was apparent that enhanced viscosity is attributed to the increased trehalose concentration. In addition, the presence of arginine salt and trehalose in spray-dried powders greatly increased solution osmolality. Thus, an even more flexible and effective approach in producing high-concentration mAb formulations is to include only a carbohydrate sugar at a molar ratio (sugar:mAb) of <150:1, or preferably <50:1 in cases where cold storage (2–8 °C) is allowed. The arginine-containing diluent can be used to reduce liquid formulation viscosity during spray-dried powder reconstitution.
Viscosity and Osmolality of Liquid Formulations Reconstituted from Spray-Dried Powders
Conclusions
This study successfully demonstrated the scale-up of the spray-drying process and the supreme advantages of producing high-concentration/high-viscosity mAb formulations based on spray-dried powder reconstitution. The spray-dried formulations maintained good mAb stability/potency during long-term storage even with mAb:trehalose molar ratios significantly greater than 1:150. This low-sugar concentration benefited spray-drying process yield and enabled lower solution viscosity upon reconstitution. There is no process limitation in solution viscosity when high-concentration mAb formulations are prepared from spray-dried powder reconstitution compared with concentration via the conventional UF process.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2015