Abstract
A simulating leaching (migration) study was performed on a model container-closure system relevant to parenteral and ophthalmic drug products. This container-closure system consisted of a linear low-density polyethylene bottle (primary container), a polypropylene cap and an elastomeric cap liner (closure), an adhesive label (labeling), and a foil overpouch (secondary container). The bottles were filled with simulating solvents (aqueous salt/acid mixture at pH 2.5, aqueous buffer at pH 9.5, and 1/1 v/v isopropanol/water), a label was affixed to the filled and capped bottles, the filled bottles were placed into the foil overpouch, and the filled and pouched units were stored either upright or inverted for up to 6 months at 40 °C. After storage, the leaching solutions were tested for leached substances using multiple complementary analytical techniques to address volatile, semi-volatile, and non-volatile organic and inorganic extractables as potential leachables.
The leaching data generated supported several conclusions, including that (1) the extractables (leachables) profile revealed by a simulating leaching study can qualitatively be correlated with compositional information for materials of construction, (2) the chemical nature of both the extracting medium and the individual extractables (leachables) can markedly affect the resulting profile, and (3) while direct contact between a drug product and a system's material of construction may exacerbate the leaching of substances from that material by the drug product, direct contact is not a prerequisite for migration and leaching to occur.
LAY ABSTRACT: The migration of container-related extractables from a model pharmaceutical container-closure system and into simulated drug product solutions was studied, focusing on circumstances relevant to parenteral and ophthalmic drug products. The model system was constructed specifically to address the migration of extractables from labels applied to the outside of the primary container. The study demonstrated that (1) the extractables that do migrate can be correlated to the composition of the materials used to construct the container-closure systems, (2) the extent of migration is affected by the chemical nature of the simulating solutions and the extractables themselves, and (3) even though labels may not be in direct contact with a contained solution, label-related extractables can accumulate as leachables in those solutions.
- Extractables
- Leachables
- Simulation studies
- Parenteral and ophthalmic drug products
- Leaching (migration) study
Introduction
Most pharmaceutical products are packaged in a container-closure system to preserve and protect the product during its manufacturing, distribution, storage, and use. During this period of contact, the pharmaceutical product and its packaging system can interact, potentially affecting product quality and safety. For example, substances in the container-closure system can leach from the system and become entrained in the product, thus becoming foreign impurities (leachables) in the product. The need to establish what effect, if any, that such foreign impurities have on product quality and safety is well established in the regulatory literature (1⇓–3), and the means of establishing the effect have been the focus of numerous authoritative texts on the subject (for example, 4⇓⇓⇓–8).
A comprehensive strategy for assessing the potential safety and quality risk posed by such foreign impurities generally involves three stages: (1) materials characterization, which is the procurement of knowledge about the composition and general properties of a container-closure system's materials of construction, (2) extractables profiling of the container-closure system and/or its components (via a controlled extraction simulation study), and (3) as necessary and appropriate, leachables profiling of the pharmaceutical product (leachables migration study). Material characterization establishes those chemical entities (including additives and ingredients) that are present in the material and that may be extracted from the material, thereby creating a basis for the selection (and justification) of appropriate materials of construction. The controlled extraction simulation study establishes which container-closure system extractable profile is relevant to the clinical use of the pharmaceutical product (and thus which closely mimics the pharmaceutical product's leachables profile). Thus, the controlled extraction simulation study can assist in materials and packaging component selection as well as contribute to the packaging system's quality and safety impact assessment. Profiling the pharmaceutical product for leachables during the course of a long-term stability study (leachables migration study) provides additional information for quality and/or safety assessment.
In situations of challenging detection limits for leachables, and certain other circumstances, a simulation study can be used either to focus the leachables study or even in place of the leachables study. A simulation study is a type of extraction or leaching study that employs simulating solvents, which are intended to closely mimic the actual drug product vehicle and its leaching potential, and accelerated leaching conditions. Extractables, leachables, and simulation studies are described in two recent USP informational general chapters (7, 8). In addition to leachables, which can accumulate in a drug product from direct contact with the packaging system, the USP recognizes the term “migrants”, which can accumulate in a drug product after crossing a barrier (e.g., from a label through a plastic bottle).
The Product Quality Research Institute (PQRI) has been an active participant in the effort to develop effective, science-driven, and risk-based strategies for the safety qualification of container-closure systems for pharmaceutical products. For example, in 2006, PQRI issued the report, “Safety Thresholds and Best Practices for Extractables and Leachables in Orally Inhaled and Nasal Drug Products”, which provides a scientific rationale and process to address packaging systems use for orally inhaled and nasal drug products (OINDPs) (4). This report includes best demonstrated practices for addressing extractables and leachables, specifically relevant to the OINDP dosage forms. More recently, the PQRI expanded its efforts to include parenteral and ophthalmic drug products (PODPs). One aspect of the PQRI PODP activity was to develop and examine the three-stage approach discussed previously. To this end, the PQRI PODP Leachables and Extractables Working Group initiated a study that generated and interpreted data from controlled extraction studies performed on multiple polymeric and elastomeric materials of construction commonly encountered in PODP packaging systems (stages 1 and 2). In this study, the test materials were subjected to different extraction conditions and the resulting samples (i.e., extracts) were then characterized for extracted substances to establish the materials' general extractables characteristics (9).
Continuing its assessment, the PQRI PODP team performed a second study to address the third stage, the leaching (migration) study, via a controlled simulation study (10). In this study, a model container-closure system, more or less consistent with systems used with PODPs, was constructed from either materials that the PODP team had characterized in its previous study or that were characterized as part of this study. This model container-closure system was then filled with simulating solvents relevant to liquid PODP dosage forms and the filled units were subjected to a leaching (migration) study.
The purpose of this report is to discuss the design and results of the PODP leaching (migration) study, specifically considering the role of such a study in the safety assessment of a container-closure system used for PODP products.
Experimental
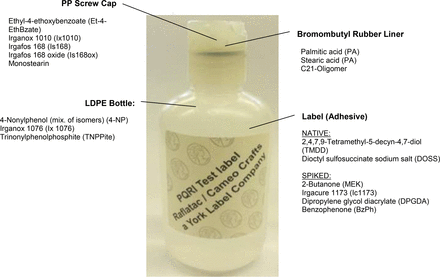
Test Article (see Figure 1)
The individual components of the model container-closure system. The bottle used is shown with the label already applied. The major ingredients or extractables from the individual components of the test system are listed. The foil overpouch, used primarily as a barrier to reduce solvent loss from the test article, is not shown.
The model container-closure system consisted of a low-density polyethylene (LDPE) bottle (4 oz Natural LDPE, Boston Round Bottle; Container & Packaging Supply, part B347A), a polypropylene (PP) cap (20-410 Natural Smooth Disc Top; Container & Packaging Supply, part L764), an adhesive label (UPM Raflatac), a rubber gasket (brominated isobutylene isoprene copolymer), and a foil overpouch (Figure 1). The components of the model container-closure system were used as received.
Leaching (Migration) Simulation Study
General:
The test units for the leaching (migration) study were prepared as follows. Individual bottles were filled with extraction solvent (nominal 100 mL), a rubber gasket was inserted on the top of the neck of the bottle, and the cap was tightly screwed down on the gasket. Spiked labels were manually applied to the outer surface of the bottles and the labeled bottles were placed in a foil pouch, which was subsequently closed by heat-sealing.
The leaching (migration) study was performed twice. The first study, with relatively fewer and later time points, served the purpose of range finding. The results of this range finding study facilitated the optimization of the experimental design. The second study, with relatively more frequent and earlier time points, reflected the optimized experimental design. Because the leaching (migration study) employed simulating solvents (as opposed to a drug product) and accelerated storage conditions, it is properly termed a simulation study.
Leaching Solvents:
The leaching solvents were as follows:
Water pH 2.5: A salt/acid solution was prepared containing 0.01 M KCl and 0.003 M HCl. The pH was adjusted to 2.5 as needed.
Water pH 9.5: A buffer solution was prepared containing 0.066 M dibasic sodium phosphate and monobasic 0.0045 M sodium phosphate. The pH was adjusted to 9.5 with 1 N NaOH.
50:50 isopropanol (IPA) and water: Equal volumes of IPA and water were mixed. This solution is referred to as IW in certain places in this manuscript.
These leaching solvents were chosen as PODPs are primarily aqueous and formulated between these pH extremes. The 1:1 IPA/water mixture represents a PODP that contains an organic surfactant or solubilizing agent.
Leaching Conditions:
Leaching was conducted at 40 °C (humidity not controlled) for up to 6 months. These leaching conditions were chosen because they are generally accepted to be the proper acceleration of a 2-year ambient temperature product shelf-life. In the range-finding study, test units were stored in either an upright or inverted configuration, as the inverted configuration results in direct contact between the rubber gasket and the fill solution. The optimized study included only inverted test units.
The time points for the range-finding study included 1, 2, and 6 months of storage. The time points used in the optimized study included 0.75, 2, 5, 12, 25, 70, 98, 105, and 180 days of storage.
Label Spiking:
Four compounds were spiked onto the bottle-contacting surface of the label prior to placing the label onto the bottle. These four compounds (see Table I) were chosen because they possess a range of physiochemical properties and are known substances associated with labels. The labels were spiked with these substances so that the pool of label-related extractables was sufficiently high that they could be effectively quantified in the fill solutions.
Label Spiking Compounds Employed in the Leaching (Migration) Study
Spiking was accomplished as follows: For the range-finding study, a mixed standard of the spiking compounds was prepared at a nominal concentration of 250 mg/mL, except for methyl ethyl ketone (MEK), which was used as the dilution solvent (at a level of approximately 615 mg/mL). Immediately before placing the label on the bottle, two drops of 2.5 μL each of the mixed standard spiking solution were placed onto the back of the label on the adhesive layer. The label was then immediately pressed and sealed to the filled bottle, followed by sealing the labeled bottle in the foil pouch.
The spike level was chosen so that spiked substances could be readily detected if migration occurred. A 5 μL spike of a 250 mg/mL solution equates to a spike amount of 1250 μg for the semi-volatile compounds and 3080 μg for the MEK. If a 10% migration rate was obtained in this study, then the resulting concentration of the compounds in the extraction solvents would be 1.25 μg/mL for each semi-volatile target [1250 μg × 0.10 (10% migration)/100 mL (total solution volume) = 1.25 μg/mL] and 3.08 μg/mL for MEK. Such accumulation levels were detectable and within the operating range of the analytical techniques used in this study.
An alternate spiking process was used for the optimized study. In this case, the spiking standard was prepared to contain approximately 50 mg/mL of each spike compound, using polyethylene glycol (PEG) 200 as the solvent. A 10 μL aliquot of the viscous spiking standard was evenly distributed on the inner adhesive surface of the label, which was immediately placed on a filled bottle, which in turn was immediately sealed into a foil overpouch. Assuming a migration rate of 100%, this spike would result in a fill solution containing approximately 5 μg/mL of each spike compound.
Solvent Blanks:
Solvent blanks were obtained by storing portions of unused leaching solvent in inert containers (glass for pH 2.5 and IPA/water, Teflon for pH 9.5).
Leachate Analysis, Range Finding
Test Methods:
The leachates (and solvent blanks) were analyzed for organic and inorganic extractables (simulated leachables) using methods that are typically employed for the purpose of extractables screening. Generally speaking, this included gas chromatography with headspace sampling and mass spectrometric detection (HS-GC-MS) for volatile substances, direct injection GC-MS for semi-volatile substances, and high-performance liquid chromatography with both UV absorption and mass spectrometric detection (HPLC-DAD-MS) for non-volatile substances. Extractables (simulated leachables) were also measured based on their elemental constituents via inductively coupled plasma mass spectrometry (ICP-MS).
Sample Processing Prior to Analysis:
Solvent switching was performed on all leachates intended for GC-MS analysis. A portion of each leachate was liquid-liquid extracted with an equivalent volume of methylene chloride. The organic layer was analyzed directly. For HS-GC-MS and HPLC-DAD-MS analyses all extracts were analyzed directly. For ICP-MS, the leachates were analyzed directly, except the IPA/water extracts, in which case the IPA was evaporated off prior to analysis. The ICP-MS samples were acidified prior to analysis.
Test Systems and Operating Conditions:
As the purpose of this study was range finding, the specific analytical instruments and test method operating conditions used in the chromatographic analyses performed in this study are not fully reported as the instruments, methods and operating conditions used in the optimized study are more relevant. As the ICP-MS analyses were only performed during range finding, the analytical details for these analyses are shown in Table II.
Instrument Parameters; ICP-MS
Extract Analysis, Optimized Study
Test Methods:
The leachates (and solvent blanks) were analyzed for targeted organic extractables using appropriate gas chromatographic (GC) and liquid chromatographic (LC) methods developed for this purpose.
Sample Processing Prior to Analysis:
Samples (leachates and blanks) were prepared for the GC analysis by transferring 200 μL of the sample, 100 μL of an internal standard solution (39 μg/mL 2-fluorobiphenyl in methanol), and 800 μL isopropanol into a 2 mL sample vial. The vials were capped and vortexed briefly. Samples were prepared for LC analysis by transferring 100 μL of an internal standard solution (106 μg/mL Irganox 415 in methanol) and 1000 μL of the extract into a 2 mL sample vial. The vials were capped and vortexed briefly.
Test Systems and Operating Conditions:
The specific analytical instruments and test method operating conditions used in chromatographic analyses performed in the optimized study are delineated in Tables III and IV.
Typical Operating Parameters, GC/MS Analyses
Typical Operating Parameters, LC/UV/MS Analyses
Results and Discussion—Range Finding
Organic Extractables
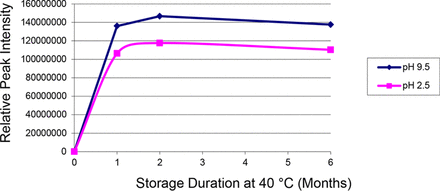
Figure 2 illustrates the leaching trends of one of the intentionally added label-related extractables (MEK). The migration of MEK through the LDPE bottle and into the leachate occurs quite rapidly and migration is essentially complete and asymptotic levels were achieved at the first time point (1 month). Because MEK is label related, the accumulation levels of this spiked extractable were not affected by whether the filled bottles were stored upright (no direct contact between the extracting solution and the elastomeric gasket or PP cap) or inverted (direct contact between the extracting solution and the elastomeric gasket or PP cap). Similar leaching (migration) profiles were obtained for the other spiked label-related extractables.
Leaching (migration) profile for methyl ethyl ketone during the range-finding Study. The leaching of this label-related extractable was relatively rapid and was essentially completed by the first time point. The accumulation of this uncharged extractable is only marginally affected by the pH of the extracting medium.
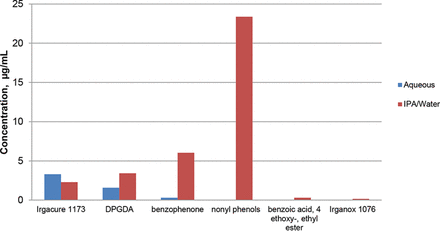
As the spiked extractables where intentionally added to the labels, it is possible to determine their extent of leaching. If 100% leaching of the spiked compounds—Irgacure 1173, dipropylene glycol diacrylate (DPGDA), and benzophenone—were to occur and the migration was strictly in the direction of the solution (and not outward through the label), a solution concentration of 12.5 μg/mL would result. Under such an assumption, the extents of leaching for Irgacure 1173, DPGDA, and benzophenone were calculated to be 26%, 27%, and 48%, respectively, in the IPA/water extracts.
The accumulation level of both label- and container-related extractables is illustrated in Figure 3. While the accumulation levels of the highly water-soluble label targets (Irgacure 1173 and DPGDA) were generally similar in the aqueous and organic solvents, the poorly water-soluble, non-polar container-related organic extractables, such as the nonyl phenol isomers, benzophenone, and Irganox 1076, only accumulated in the IPA/water leachates in measurable quantities. The accumulation levels of the label- and bottle-related extractables were not affected by whether the filled bottles were stored upright (no direct contact between the extracting solution and the elastomeric gasket or PP cap) or inverted (direct contact between the extracting solution and the elastomeric gasket or PP cap).
Maximum accumulation levels for the organic extractables measured during the range-finding study.
These data established two areas for optimization in the second study reported herein. First, it is clear that fully establishing the leaching (migration) profile would require test intervals much earlier and more frequent than were used in the range-finding study. Second, the label spiking was found to be sub-optimal. Specifically, the levels at which extractables were spiked onto the label were too high and the spiking of the label did not produce an even distribution of the spiked compounds across the surface area of the label. While the leaching of the spiked extractables was readily followed in the range-finding study, it was not possible to follow the leaching of extractables that were not spiked into the label and were intrinsic to the label as the levels of the spiked extractables were significantly greater than the levels of the intrinsic extractables. Furthermore, the leaching of the spiked compounds was mechanistically constrained by their uneven distribution on the label. Thus, the spiking levels were reduced and the spiking process was modified in the optimized study.
Additionally, the leaching trends noted in the range-finding study established the approximate levels to which the extractables accumulated in the fill solutions. Knowing the approximate accumulation levels facilitated the development of analytical methods that had the necessary sensitivity, specificity, accuracy, and precision to support the target extractables testing that was used in the optimized leaching (migration) study.
Elemental Extractables
While the ICP-MS testing was performed a using a periodic table scan of 70 elements (see Table II for the list of elements included in the scan), extractables containing most of these elements were not detected in any of the leachates. Those elements that were present in the leachates at measurable levels included B, Mg, Al, Si, K, Ca, Ti, Zn, and Br. The likely source of most of these elements is the elastomeric gasket: B and Br from the rubber itself, Ti from the titanium dioxide used as a colorant, Ca and Zn as counter-ions in the fatty acid salts used as lubricants, Al and Si from the elastomer's calcined aluminum silicate, and Mg from the material's calcined magnesium oxide.
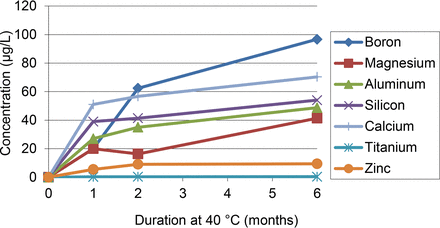
The following observations were made concerning the leaching behavior of the elemental extractables (see Figures 4 and 5):
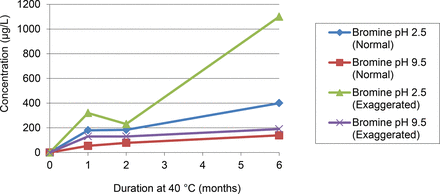
The element-containing extractables were leached in higher quantities at lower pH.
The levels of element-containing extractables were larger, inverted (exaggerated) versus upright test units. This is consistent with the elastomeric gasket being the source of these extractables, as the gasket is in direct solution contact in the inverted configuration.
Complete leaching of the element-containing extractables was not achieved under the conditions of this study. While the leaching plots typically reflect an approach to asymptotic concentrations, the asymptotic behavior was not achieved for most element-containing extractables and thus the levels of these extractables would continue to increase for longer storage durations. Nevertheless, the most rapid leaching of the metallic extractables occurred in the early stages of the extraction study (less than 1 month contact). Conversely, the leaching of the non-metallic extractables (B and Br) at low pH did not achieve asymptotic behavior over the course of the study, suggesting that either (1) the pool of these extractables is larger than the pool of the metallic extractables, (2) the mechanism of leaching is different than the metallic extractables, or (3) a combination of both.
Leaching profiles for elemental extractables, range-finding study, pH 2.5.
Leaching profiles for bromine, range-finding study.
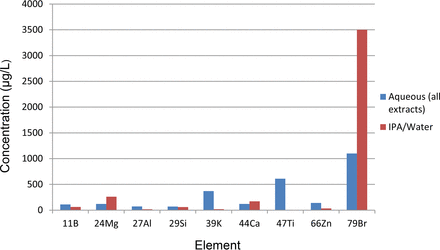
Figure 6 documents the maximum levels of the element-containing extractables, aqueous versus IPA/water leaching solutions. In general, the metallic extractables form two groups with respect to their relative leached levels, aqueous leachates versus IPA/water leachates. One group, reflecting Al, K, Ti, and Zn, accumulated at higher levels in the aqueous leachates (more specifically, the low-pH aqueous leachates). A second group, including Ca, Mg, and Si, accumulated at either similar or higher levels in the IPA/water leachates. It is likely that this different behavior reflects a different leaching mechanism. For example, it is likely that the element-containing extractables that accumulated to higher levels at the low-pH aqueous leachates were leached from the test article via a process of either ion exchange or dissolution of an inorganic oxide. On the other hand, the metallic extractables that accumulated to higher levels in the IPA/water leachates were leached from the test article in the form of an organic compound that is more soluble in IPA/water than it is in water.
Maximum accumulation levels the elemental extractables measured during the range-finding study.
Unlike the metallic extractables, Br-containing extractables accumulated at much higher levels, IPA/water versus aqueous. This behavior could reflect the leaching of Br-containing hydrocarbon oligomers by the IPA/water extraction medium.
Results and Discussion—Optimized Study
Establishing Targeted Extractables
It is reasonable and appropriate to suggest that there be a relationship between (1) the ingredients intentionally added to a material of construction, (2) the extractables that are revealed when the material (or a component or system containing the material) is characterized by a controlled simulation study, and (3) the leachables that are present in a pharmaceutical product stored in the system constructed from the material. Going forward, one would expect that the ingredients would be indicative of the extractables and that the extractables would be indicative of the leachables. In the reverse direction, one would expect that leachables would be largely explainable on the basis of extractables and that extractables would be largely explainable on the basis of ingredients. In fact, these observations are the basis of extractables and leachables correlations. Furthermore, these observations establish why a three-stage assessment process including material selection (based on ingredients), system qualification (based on extractables), and product assessment (based on either leachables or simulating extractables) is an appropriate means of developing and qualifying safe and effective packaged pharmaceutical products.
Information that can guide the selection of target extractables in the optimized leaching (migration) study is contained in Tables V through VII. Specifically, Table V contains compositional information that was obtained from the vendors of the materials that made up this study's test system while Table VI contains the extractables profiles that were obtained for these materials via controlled extraction studies. Table VII qualitatively reconciles the compositional and extractables information and uses this information to select those extractables that were targeted in the optimized leaching (migration) study. It should be noted that these targeted substances are termed targeted extractables because the optimized leaching (migration) study is a simulation of the actual clinical use of the test system. Had this leaching (migration) study been performed with a specific drug product under the actual conditions of storage and use of that drug product, then the targeted substances would have been termed target leachables.
Vendor Information Provided about the Composition of the Materials Used in the Tested Container-Closure System
Extractables Profiles: Materials of Construction, Established via a Controlled Extraction Study
Reconciliation of Ingredients, Extractables, and Targeted Extractables
Extractables Targeted in the Optimized Study, Label-Related
Extractables Targeted in the Optimized Study, Cap-Related
Extractables Targeted in the Optimized Study, Gasket-Related
Extractables Targeted in the Optimized Study, Bottle-Related
As seen in Table VII, the extractables profiles for the test system's individual materials of construction can be well-correlated to the materials' composition, known or speculated. For example, while the vendor of the polyethylene bottle did not provide its compositional information, it is reasonable to suspect that the polyethylene was formulated to contain one or more antioxidants, and in fact commonly employed antioxidants were present in the material's extractables profile. Considering a material whose composition was well known, the rubber material's extractables can generally be linked to its intentional ingredients.
Several factors are relevant when selecting extractables to target in a leaching (migration) study. Generally speaking, the overriding factor for the selection of target substances (either extractables or leachables) is the potential for the substance to adversely affect some quality attribute (including safety) of either the pharmaceutical product or the packaging system. Given that performing an impact assessment was not within the scope of this study, this means of selecting targets was not used. Rather, the targets noted in Table VIII were chosen based on secondary considerations, including:
measured level in the leachates (based on the generalization that the extractables at the highest levels tend to have the greatest impact potential and are the most readily measurable),
analytical expediency, and
ability to represent a particular compound class or a particular source ingredient.
Leaching Profiles for the Label-Related Targeted Extractables
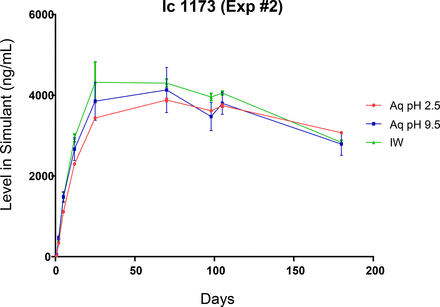
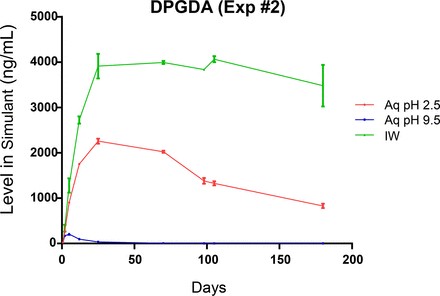
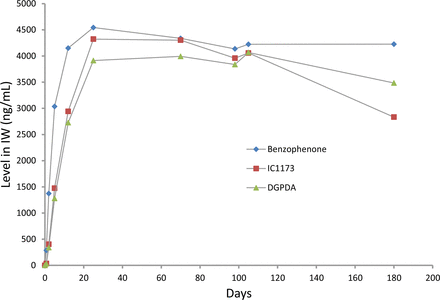
Leaching profiles for the three spiked, label-related target extractables are shown in Figures 7 through 9. The profiles of the individual extractables are both similar and dissimilar, consistent with the chemical nature of the extractable and the extracting matrix. As all three extractables are highly soluble in the IPA/water extraction medium, it is to be expected that all three extractables would have similar leaching profiles in this medium. This is the case, as shown in Figure 10, which indicates that the leaching of these three extractables is essentially complete after approximately 20 days of storage, at which point an equilibrium level is achieved. This equilibrium level, approximately 4200 ng/mL, is comparable to the total pool of these spiked substances, which was approximately 5000 ng/mL.
Leaching profile of a spiked label extractable, Irgacure 1173, optimized study. The leaching of this non-ionic, highly water soluble and stable extractable is similar in all three leachate media.
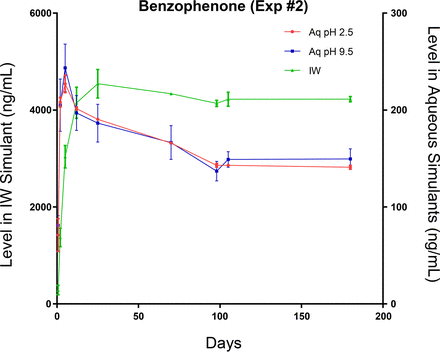
Leaching profile of a spiked label extractable, benzophenone, optimized study. The different leaching behavior of this extractable in the aqueous leachate matrices likely reflects its limited aqueous solubility.
Leaching profile of a spiked label extractable, dipropylene glycol diacrylate (DPGDA), optimized study. The different leaching behavior of this highly water-soluble, non-ionic extractable in the aqueous leachate matrices likely reflects its acid- and base-catalyzed degradation.
Leaching profile of three spiked label extractables in isopropanol/water, optimized study. All three spiked extractables behave similarly in this leachate matrix, achieving an equilibrium state after roughly 20 days of storage.
While the behavior of these three extractables is similar in the IPA/water medium, their behaviors are very different in the aqueous extracts. As Irgacure 1173 is both highly water soluble and non-ionic, its leaching is very similar in all three leachate matrices (Figure 7). As benzophenone is less water soluble, its equilibrium level in the aqueous leachates is lower than its equilibrium level in the IPA/water leachate (Figure 8).
The leaching profiles obtained for DPGDA (Figure 9) are quite different than the profiles for the other spiked label-related extractables, as the profiles are quite different in all three leachate media. Because this extractable is both highly water soluble and non-ionic, it is reasonable to expect that it would exhibit similar behavior to Irgacure 1173, where the profiles in all three leachate media are essentially the same. The lower and decreasing levels observed in the pH 2.5 and especially the pH 9.5 leachates suggest that this extractable is degraded by an acid- and base-catalyzed process.
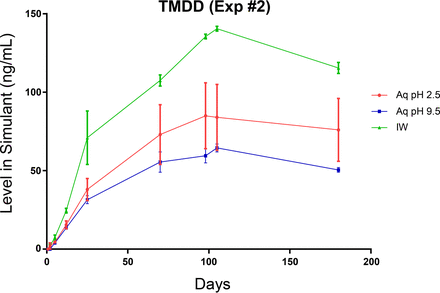
The behavior of a fourth label-related extractable, 2,4,7,9-tetramethyl-5-decyn-4,7-diol, is shown in Figure 11. This targeted extractable differs from the spiked targets in that (1) this extractable is native to the label, and not artificially spiked as were the previously discussed targets and (2) it is a degradation product of, or impurity in, one of the constituents of the label's adhesive (Surfynol PSA 336). As a degradation product, its leaching would be slower than the spiked extractables because its leaching rate reflects the combination of its production rate and its diffusion rate whereas the leaching rate of the spiked extractables reflects solely the diffusion rate. Nevertheless, this extractable achieves its equilibrium level in solution after approximately 105 days of storage. Because this non-ionic extractable has a low water solubility, its equilibrium level in the aqueous leachates is similar in both the low- and high-pH media but lower than its equilibrium level in the IPA/water medium.
Leaching profile of a native label extractable, 2,4,7,9-tetramethyl-5-decyn-4,7-diol (TMDD), optimized study. The different leaching behavior of this extractable in the aqueous extraction matrices likely reflects its limited aqueous solubility.
Leaching Profiles for the Cap-Related Targeted Extractables
Due to the nature of the test system, the cap is an indirect solution contact component of the system. This observation is significant as it is the explanation for the behavior of the cap-related target extractables. Although Irganox 1010, Irgafos 168, and Irgafos 168 oxide were targeted, these substances did not accumulate in the extraction media at any point in the migration study. As these substances are large molecules with low volatility, their migration from the cap and through the barriers (either the bottle or the liner) was anticipated to be very slow. Additionally, their solubility in aqueous solutions is very low, and even if they did migrate to the point where they achieved solution contact, they would not dissolve in the aqueous media to any measurable extent.
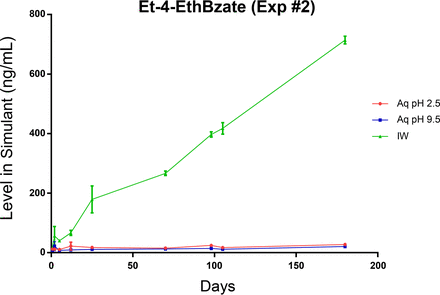
On the other hand, ethyl-4-ethoxybenzoate was readily measurable in the IPA/water leachate throughout the course of the leaching (migration) study (Figure 12). As this compound is smaller, more soluble, and more volatile than the other cap-related targets, it is reasonable to expect that it would migrate faster than the other targets. In fact, the chemical properties of this target are similar to those of benzophenone, a spiked label-related target, and thus it is reasonable to expect that the ethyl-4-ethoxybenzoate would have the ability to migrate out of the cap, through the bottle, and into the fill solution. Of course, such a process for ethyl-4-ethoxybenzoate would be slower than for benzophenone, given ethyl-4-ethoxybenzoate's longer, and likely more complex, migration pathway.
Leaching profile of a cap-related extractable, ethyl-4-ethoxybenzoate, optimized study. The significantly lower levels of this extractable in the aqueous versus the IPA/water leachates are a manifestation of this compound's propensity to partition into the various components of the container-closure system. Thus, it leaches from the cap and then preferentially partitions into the other system components (e.g., bottle) as opposed to the fill solution.
As shown in Figure 12, ethyl-4-ethoxybenzoate does not accumulate in the aqueous leachates to appreciable levels. Although this behavior does not reflect the relatively high aqueous solubility of this compound (which is greater than 300 mg/L), it could be explained by either acid- and base-catalyzed decomposition of the benzoate, the small surface area of contact between the fill solution and the cap, and/or the partitioning of this compound into the other components of the container-closure system, such as the bottle.
Leaching Profiles for the Gasket-Related Targeted Extractables
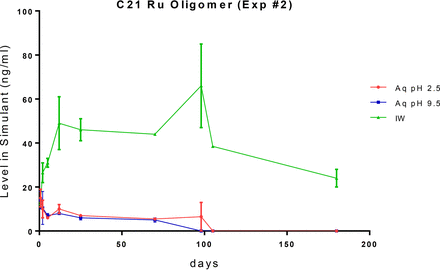
The leaching profiles of the single measured gasket-related extractable, the C21 oligomer, are shown in Figure 13. This oligomer accumulates in the fill solutions at very low levels, even in the case of the IPA/water fill solution. This behavior suggests that the various container-closure system components have a strong affinity for this oligomer and thus that the oligomer partitions primarily into these components, thereby limiting its accumulation in the fill solutions.
Leaching profile of a gasket-related extractable, C21 rubber oligomer, optimized study. The leaching behavior of this extractable reflects its limited aqueous solubility and strong partitioning into the various components of the container-closure system.
Leaching Profiles for the Bottle-Related Targeted Extractables
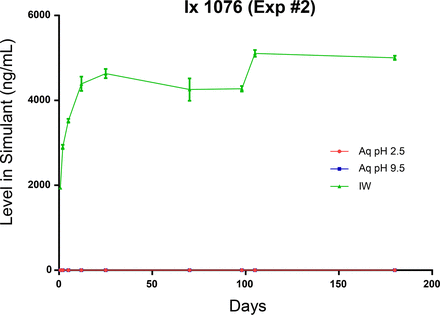
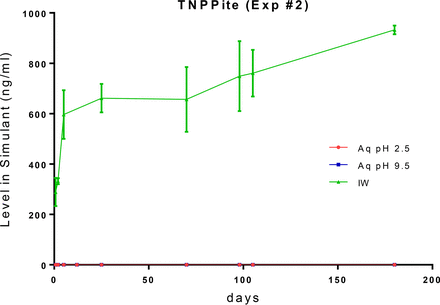
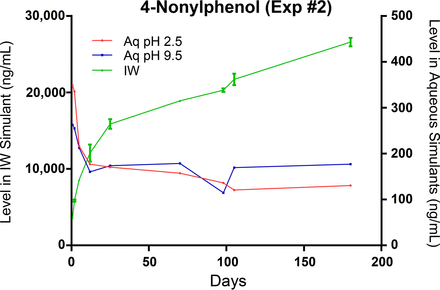
Due to the large contact surface area between the bottle and the leaching solution, relative to the other components of the test system, and their relatively large total pools in the bottle, it is reasonable to expect that bottle-related extractables would quickly accumulate in the fill solutions in readily measurable quantities. This expectation is realized for the three targeted bottle-related extractables in the IPA/water leachate, as shown in Figures 14 through 16. Given the very limited aqueous solubility of Irganox 1076, it is expected that its accumulation in the aqueous leachates would be low, and this was the outcome that was observed. The low accumulation levels of trinonylphenylphosphate in the aqueous leachates reflect both the limited aqueous solubility of this compound and its hydrolytic degradation in aqueous solutions. Given its higher aqueous solubility, 4-nonylphenol accumulated at measurable concentrations in the aqueous leachates, and as it is non-ionic there was no difference in its leaching behavior as a function of extraction solution pH. However, its relatively low accumulation levels in the aqueous leachates and the rapidity with which the plateau in the leaching (migration) profile is achieved indicate that 4-nonylphenol partitions preferentially into the bottle material, as opposed to partitioning into the fill solution.
Leaching profile of a bottle-related extractable, Irganox 1076, optimized study. The different leaching behavior of this extractable in the aqueous leachate matrices likely reflects its very limited aqueous solubility.
Leaching profile of a bottle-related extractable, trinonylphenylphosphite, optimized study. The low accumulation levels of this extractable in the aqueous leachate matrices reflect both its limited aqueous solubility and its hydrolytic degradation in aqueous solutions.
Leaching profile of a bottle-related extractable, 4-nonylphenol, optimized study. The significantly lower levels of this extractable in the aqueous versus the IPA/water leachates is a manifestation of this compound's propensity to partition into the various components of the container-closure system.
Conclusions
The results of the simulated leaching (migration) study performed on a model container-closure system that is more or less relevant for parenteral drug products supports the following generalizations:
The extractables profile revealed by performing such a simulation study on a packaging system can qualitatively be correlated with compositional information obtained for the system's materials of construction.
The chemical nature of the extracting/leaching medium can markedly affect the extractables and the leachables profiles.
The chemical nature of the extractable itself can markedly affect its leaching (migration) profile.
An extractable's leaching (migration) profile reflects the interplay between the chemical properties of the leaching medium and the extractable (as per 2 and 3 above).
Element-containing extractables are leached from materials in different forms and by different mechanisms, depending on the chemical nature of the leaching medium and the chemical form of the extractable's source material.
While direct contact between a drug product and a system's material of construction may exacerbate the leaching of substances from that material by the drug product, direct contact is not a prerequisite for extraction to occur. Thus label-related and certain cap-related extractables were able to breach the barrier provided by the LDPE bottle and accumulate in the leaching solutions. Furthermore, while gasket-related extractables accumulated to higher levels when the model packaging system was stored inverted versus upright, some leaching was noted even in the upright (non-direct solution contact) configuration.
LDPE is a poor barrier to volatile and semi-volatile extractables, given that this study established that the migration and leaching of label-related extractables was quite rapid.
Furthermore, this study established that a simulated leaching (migration) study can produce an extractables profile that could reasonably be expected to mimic the leachables profile of a packaged pharmaceutical product.
Conflict of Interest Declaration
The authors(s) declare that they have no competing interests, either as representatives of their individual organizations or as volunteer members of the PQRI Leachables and Extractables Working Group.
- © PDA, Inc. 2017