Abstract
Flexible medical devices are primarily made of plasticized polyvinyl chloride (PVC). In recent times, to avoid undesired migration of the PVC plasticizers, ethyl vinyl acetate (EVA) and polypropylene (PP) has replaced PVC. Nevertheless, other additives are necessary to generate useful polymeric materials. Metallic species present in such additives can also leach out into the infusion solutions. The migration of barium (Ba), cadmium (Cd), lead (Pb), tin (Sn), and zinc (Zn) from devices made from PVC, EVA, and PP was evaluated. Bags and infusion sets were decomposed and their metallic contents analyzed. Glucose, NaCl, and Tween 80 were assessed as extraction media. These solutions were stored in PVC, EVA, and PP bags, heat-sterilized, and stored for 8 months at room temperature. Aliquots were taken before and after sterilization and then once per month to determine the contents of the metals. Commercial glucose and NaCl infusions were analyzed by taking aliquots of the solutions from the bags and from the administration set after their administration to patients. The three polymers contained the five metals. Ba was found in the highest concentration in all samples, with a mean of 8.0 mg/kg in PVC, 4.2 mg/kg in EVA, and 4.7 mg/kg in PP samples. Despite this, the only element that migrated into the glucose, NaCl, and Tween 80 solutions was Zn. The same result was found for the commercial glucose and NaCl infusions. Moreover, the Zn concentration in the administration sets was on average 52% higher than that found in the bags.
LAY ABSTRACT: Flexible medical devices for infusions and artificial nutrition are made of plastics, such as polyvinyl chloride (PVC), ethyl vinyl acetate (EVA), and polypropylene (PP). These polymers contain additives necessary to generate useful materials. Metallic species present in these additives can leach out into the infusion solutions and come into contact with patients. To assess the risk of patient exposure to these metals, we evaluated the migration behavior of barium (Ba), cadmium (Cd), lead (Pb), tin (Sn), and zinc (Zn) from devices made from PVC, EVA, and PP. Bags and infusion sets were analyzed. Glucose, NaCl, and Tween 80 were investigated as extraction media. The three polymers contained the five metals. Ba was found in the highest concentration in all samples. Despite this, the only element that migrated into the glucose, NaCl, and Tween 80 solutions was Zn.
Introduction
The use of additives in polymeric materials is generally required to make them useful as containers. The essential additives for all plastic materials serve as stabilizers and lubricants, and in the case of flexible polyvinyl chloride (PVC), plasticizers are also incorporated. Other additives that may be used include fillers, processing aids, impact modifiers, and pigments (1).
The debate over the use of PVC in flexible medical devices focuses on whether the risk of exposure to additives that can leach during use is significant enough to warrant restrictions on the use of this plastic. Soft PVC contains up to 50% (w/w) plasticizers, usually phthalate esters, which are not chemically bound to the plastic and therefore may leach or migrate (2). Owing to toxicological concerns with the migration of phthalates (3, 4), polyolefins and ethyl vinyl acetate (EVA) have been used as replacements for PVC in medical devices. The properties of EVA, a random copolymer, depend on the vinyl acetate (VA) content and on its molecular weight. EVA with a VA content of 3% to 12% has a similar flexibility to that of plasticized PVC. Polyolefins are more versatile, and their properties can be tuned without the use of plasticizers (5). Nevertheless, other additives required to confer other properties could be present in these polymers.
Thermal and light stabilizers ensure safe production and processing of plastics and rubbers and protect products against premature aging and weathering owing to the direct or indirect impact of heat and ultraviolet light. The effectiveness of stabilizers depends on their solubility, ability to stabilize in different polymer matrix, and the distribution in the matrix, among others (6).
Among the potential additives, metal compounds can be used to stabilize polymeric materials. The major metals contained in stabilizers are lead (Pb), barium (Ba), calcium (Ca), zinc (Zn), and tin (Sn). The stabilizers are classified into Pb stabilizers, Ba-Zn stabilizers, Ca-Zn stabilizers, and Sn stabilizers (7). They may be used directly or by combinations.
Lead stabilizers are tetra-basic lead sulfate, tri-basic lead sulfate, di-basic lead phosphite, di-basic lead phthalate, di-basic lead stearate, and neutral lead stearate. The cost/performance ratio and physical properties have made them the materials of choice for many applications. Ba-Zn stabilizers and Ca-Zn stabilizers are used as metallic soaps, such as stearates or laurates. PVC compounds incorporating Ba-Zn stabilizers are most commonly used in flexible materials. Sn stabilizers can be divided into two main groups, the first based on tin–oxygen compounds (Sn carboxylates) and the second tin–sulfur compounds (Sn mercaptides). The Sn-mercaptides are usually mixtures of di-alkyl and mono-alkyl tin compounds. Some Sn-based stabilizers are approved for use in food contact applications and for use in rigid medical applications. In addition, these metals can be used in liquid mixed metal stabilizer systems. They are based on Ba, Zn, Ca, magnesium (Mg), or potassium (K) carboxylates (6).
Environmental concerns on the use of these stabilizers have increased owing to their toxic metal content. In Europe, Pb stabilizers are increasingly replaced by other types, for example, Ca-Zn stabilizers. Cadmium-based systems have been available for many years owing to their excellent performance. However, their use in the European Union was phased out on a voluntary basis as part of the industry's Voluntary Commitment of 2000 (8).
Calcium-based stabilizers are now largely used. Ca-Zn stabilizers have been used as alternatives to Sn-mercaptides and Sn-carboxylate. Similarly, Ca-based stabilizers are now an alternative to liquid mixed metals for several flexible applications.
Additives authorized by the European Pharmacopoeia include zinc octanoate, zinc stearate, and zinc oxide, which are a plasticizer, lubricant, and filler, respectively. Magnesium oxide and calcium stearate may also be used as lubricants and fillers (9).
Additives containing metals such as Pb, Sn, and Ba, although useful for polymers in general, are not allowed in the materials for plastic containers destined for pharmaceuticals use. Nevertheless, Zn, Pb (10⇓–12), Ba (13), and cadmium (Cd) (11, 12) have all been found in solutions for parenteral nutrition, but their source was not identified. The leaching of metals into pharmaceutical products stored in containers made of polymeric materials containing additives is possible but has not been well characterized. Moreover, the latest International Council for Harmonization guideline for Elemental Impurities, ICH Q3D, includes Cd, Pb, Ba, and Sn as impurities to be controlled in new drug products (14).
The presence of elemental impurities in solutions for parental administration is a problem because of the toxicity of some elements and the method of administration, which bypasses the gastric barrier. Although these impurities can exist in the ingredients of parenteral nutrition, the primary container is the most important source of contaminants in pharmaceutical products (15).
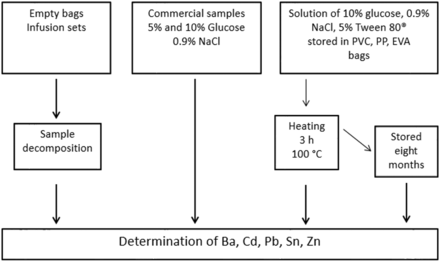
In this study, we evaluated the presence of metallic elements that can be used as additives for polymeric materials and compared their contents in PVC, EVA, and polypropylene (PP) bags and infusion sets for parenteral use. We investigated the presence of Ba, Cd, Pb, Sn, and Zn in these container materials, as they are the usual metals present in the polymer stabilizers. We also explored the influence of formulation composition, storage time, and heat (sterilization process) on the metal migration rates. NaCl, glucose, and Tween 80 were used as the extracting media during the storage period and during heat sterilization. Finally, the metals leached into commercial infusions of NaCl and glucose solutions were analyzed. A scheme in Figure 1 summarizes the analyses carried out in this study.
Scheme of the steps carried out in this study.
Experimental
Instrumentation/Procedure
Measurements were carried out using a Model contrAA® 700 high-resolution continuum source atomic absorption spectrometer (HR-CS-AAS) (Analytik Jena AG, Jena, Germany) equipped with a transversely heated graphite atomizer and an MPE 60z autosampler. The integrated absorbance values (peak areas) were used to evaluate the signal. Argon (99.996%, White Martins, São Paulo, Brazil) was used as the purge gas. Atomization was conducted on pyrolytic-coated graphite tubes (with integrated platforms) from Analytik Jena AG. The instrumental parameters, operational conditions, and furnace temperature programs are shown in Tables I and II. An electric muffle (Heraeus, MR-170E), a laminar flow class-100 clean bench (Trox Technik, Curitiba, Brazil), and a Teflon sub-boiling distillation apparatus (Berghof, Eningen, Germany) were used.
Instrumental Parameters for Ba, Cd, Pb, Sn, and Zn Determination by Graphite Furnace HR-CS-AAS
Temperature Programs (°C) for the Measurement of Ba, Cd, Pb, Sn, and Zn for the Different Samples Analyzed
Reagents
All reagents were of analytical grade, and all solutions were prepared with distilled and deionized water that was further purified by a Milli-Q water purification system (electrical resistivity of 18.0 MΩ cm, Millipore, Bedford, MA, USA). The reagents used in this study were supplied by Sigma-Aldrich (St. Louis, MO, USA) and Merck (Darmstadt, Germany). Nitric acid was sub-boiling distilled prior to use. Standard solutions of Ba, Cd, Pb, Sn, and Zn were prepared by diluting appropriately 1000 mg/L stock solutions from SpecSol® (NIST, Gaithersburg, MD, USA). The chemical modifiers used in this study were palladium nitrate (2 g/L, Fluka, Switzerland), magnesium nitrate (2 g/L, Merck), ammonium phosphate 1% (m/v) (Merck), and calcium chloride 1% (m/v) (Vetec, Brazil).
Contamination Prevention
All laboratory materials were immersed for at least 48 h in a 10% (v/v) HNO3/ethanol solution and washed with Milli-Q-purified water shortly before use. To avoid contamination from the air, samples and solutions were prepared in a class 100 laminar flow clean bench. The PVC, EVA, and PP samples were cut with titanium scissors to avoid metal contamination.
Sample Collection
New, empty EVA bags (Rivero, Buenos Aires, Argentina) and PP bags (Rmdesc, Porto Alegre, Brazil) were purchased from hospital product suppliers. Because we did not find a supplier of new, empty PVC bags in Brazil, PVC bags containing infusion solutions (0.9% NaCl, 5% and 10% glucose, and peritoneal dialysis solution) were emptied, washed with ultrapure water, and left to dry under laminar flow at room temperature. Simple PVC gravitational infusion equipment from Baxter and Rmdesc were also analyzed.
Bags containing commercial infusion including their respective administration sets were collected from the neonatal intensive care unit of the Hospital of Santa Maria University. The infusion solutions were 5% glucose (PVC, Baxter, 100 mL), 10% glucose (PP, Fresenius Kabi, 250 mL), and 0.9% NaCl (PVC, Baxter, 100 mL).
Analysis of the Bag Materials
A previously reported method was used for sample decomposition (16). The PVC, EVA, and PP bags and devices were cut in small pieces (approximately 1 cm2), washed with purified water, and left to dry on a clean bench. Samples (approximately 1 g) of each material were accurately weighed in decontaminated porcelain crucibles, 1 g of sodium nitrate was added to each, and the crucibles were heated in a muffle furnace for 1 h at 300°C and then 4 h at 500°C. After cooling, 2 mL of nitric acid and 1 mL of hydrogen peroxide were added to the samples, and the obtained solutions were quantitatively transferred to 50 mL volumetric flasks. The samples were brought to the appropriate volume with water. All samples were analyzed in triplicate. A blank sample was obtained by carrying out the procedure with only 1 g of sodium nitrate in the crucible. Recovery tests were performed by separately adding a volume of each standard solution containing 2 μg of the analytes to material samples and carrying out the decomposition procedure.
Bag Content Analysis
The contents of the bags were analyzed directly without further treatment. Recovery tests were performed by adding a volume of each standard solution of the analytes to each sample to achieve a final concentration of 10 μg/L and carrying out the analysis.
Influence of Heat and Time on Metal Migration
To verify the influence of the sterilization process on the migration of the metals, aqueous solutions of glucose (10%), sodium chloride (0.9%), and Tween 80 (5%) were prepared and stored in PVC (250 mL), EVA (250 mL), and PP (500 mL) bags. The samples were placed in an oven for 3 h at 100°C to replicate the typical sterilization procedure (17). Solutions were analyzed before and after heating. Each sample was prepared in triplicate. After the sterilization procedure, samples were stored at room temperature for 8 months. Every month, a 1 mL aliquot of each sample was collected, and the contents of the analytes were measured.
Results and Discussion
Method Validation
It was not possible to use the same temperature program for all elements. The HR-CS AAS conditions for the measurements of the metals in the samples are shown in Tables I and II. Each metal required the use of a different chemical modifier (Table I). Comparing the samples, the digested polymer samples required lower pyrolysis and atomization temperatures probably because the matrix was completely calcined in the muffle furnace and then dissolved in nitric acid (Table II). The other samples required higher pyrolysis and atomization temperatures and the use of chemical modifiers. The contents of the elements were determined based on the peak volume (center pixel ± 1).
Once the appropriate temperature programs were determined, regression curves were prepared using standard calibration techniques. Calibration curves were constructed by evaluating the integrated absorbances obtained from standard solutions in the range displayed in Table III. The limits of detection (LOD) values were calculated from the equation LOD = 3.3 × Sa/b, where Sa is the intercept and b is the slope of the regression curve. The LOD values and the results obtained from the recovery assays are shown in Table III. Table III also shows the results of the recovery tests, which show the adequacy of the methods for the proposed examination.
Analytical Parameters for Determination of Ba, Cd, Pb, Sn, and Zn in the Investigated Samples
Analysis of Polymers
Table IV displays the amounts of Ba, Cd, Pb, Sn, and Zn in the polymeric materials analyzed in this study based on the different brands and formulations. Each sample, even if it was of the same formulation and brand, corresponded to a different lot.
Ba, Cd, Pb, Sn, and Zn Contents in Different Commercial PVC, EVA, and PP Samples
All metals were found in all devices. We expected Zn to be present in the highest concentrations, as allowed additives contain this metal; however, Ba was found to be present in the highest concentrations, and the other four elements were present in lower concentrations. As shown in Table IV, there is no relationship between the amount of metal leached and the brand, size, or content of the bags.
Although these values are not high and, with the exception of Ba, are within the limits defined by the European Pharmacopeia (9) for PVC and PP for parenteral preparations, they are above the limits established by the new USP <661.1> monograph, “Materials of Construction” for the same polymers (18). The monograph, which aims at qualifying and proving the suitability of a material, establishes elemental limits for each polymer that are similar to those from the European Pharmacopeia, but they do not define the applications of the polymeric material. To compare the compendia limits and the levels found in this study, Table V shows the limits and the range of each metal measured in the samples by polymer type. The polymers listed in the USP <661.1> and in the European Pharmacopeia are PP, polyethylene, cyclic olefin, and plasticized PVC. EVA is not included in either monograph.
Ba, Cd, Pb, Sn, and Zn Contents in the PVC, EVA, and PP Samples and Compendia Limits for these Metals in the Selected Polymersa
Table V shows that, although the contents of Zn and Sn were within the limits in PVC and PP samples, in the PVC samples, Ba was above the limit in 88% of the samples, and Pb was above the limit in 100% of the samples. In two of the samples, the Cd content was at the limit (0.6 mg/kg) corresponding to 8%. On the other hand, for PP, limits for only Zn, Cd, and Pb are given in both monographs. The measured values show that while Cd reached its limit of 0.6 mg/kg, Pb exceeded its limit of 0.025 mg/kg set by the USP but not the limit of 2.5 mg/kg set by the European Pharmacopeia for heavy metals, which includes Pb.
Influence of Heat and Time on Metal Migration
Despite the presence of these metals in the device and container materials, only Zn migrated into the liquid when solutions of NaCl, glucose, and Tween 80 were stored in the PVC, EVA, and PP bags and were submitted to heat sterilization (Table VI). While Zn was observed to migrate from PVC and EVA bags, no migration was observed from the PP bags. It seems that the nature of the solution affects migration. Migration followed the order glucose > Tween 80 > NaCl for both PVC and EVA.
Zn Measured in the Prepared Solutions of 0.9% NaCl, 10% Glucose, and 5% Tween 80 Stored in PVC, EVA, and PP Bags Before and After the Sterilization Procedure and After 8-Month Storage at Room Temperature
Tween 80 was chosen as an extraction media because of its use in parenteral preparations, and as it is a nonionic surfactant, it can facilitate contact with the polymer surface and boost migration. A study compared the release of bis(2-ethylhexyl) phthalate (DEHP) from PVC bags into etoposide in Tween 80 prepared in 0.9% NaCl and in 5% dextrose (19). Although no significant differences were found between the dextrose and NaCl infusion solutions, the results confirmed that Tween 80 enhances the migration of the plasticizer. Similar results were found in another study, where, by increasing Tween 80 concentration from 1% to 25%, the amount of DEHP leached into the formulation rose from a mean of 7.1 mg/L to a mean of 42.1 mg/L, after only 4-h storage at 24°C (20). However, unlike the plasticizer, Tween 80 did not increase the migration of metallic species. The results are presented in Table VI. This result shows the poor interaction of this surfactant with metallic species and corroborates with other studies on this behavior. Tween 80 failed to leach the metallic species from clear and amber Type I glasses, while ethylenediaminetetraacetic acid (EDTA), a complexing agent under the same conditions, promoted the migration of various metals in substantial amounts (21).
During the storage period of eight months after sterilization, Zn remained the only metal measured in solutions, while the others were not detected (below the LOD). Table VI displays the concentration of Zn reached in glucose, NaCl, and Tween 80 solutions after 8 months in PVC and EVA bags, as solution stored in PP did not show any Zn leaching over the course of the experiment.
Commercial Infusion Solutions
The metal contents in commercial infusions of 5% and 10% glucose and 0.9% NaCl were similar to these results. Only Zn was detected in the glucose samples, and in addition to Zn, Pb was found in the NaCl samples. Again, the Zn concentration was higher in the glucose samples than in the NaCl samples. It is interesting to note that the 100 mL 5% glucose PVC bags from Baxter contained, on average, 5.3 times more Zn than the 100 mL 0.9% NaCl PVC bags from Baxter as well.
Because the glucose solutions were administered directly to the patients, these bags were collected along with their corresponding infusion sets. These devices are made of PVC, and both the solution in the bag and the same solution after passing through the infusion set were analyzed. Table VII shows the levels of Zn in these samples. First, there is a difference in the Zn contents in the glucose solutions stored in the PVC and PP bags. The mean Zn content in the PVC bags was higher than the mean Zn concentration in the PP bags (P < 0.05, t-test). Second, after contact with the infusion set, the Zn concentration increased 16.6% and 73.6% for sets connected to the PVC and PP bags, respectively. These results show that the main source of Zn in these solutions is the container/infusion set and that the quantity of the leachates from PVC was higher than that from PP. Pb was present in very low concentrations in the NaCl samples (1.7 μg/L and 1.6 μg/L in lots 1 and 2, respectively).
Zn Measured in Commercial Solutions of Glucose and NaCl Stored in PVC and PP Bags and Zn Measured in the Bag-PVC Infusion Set
Conclusion
Bags and infusion sets made of PVC, EVA, and PP contained Ba, Cd, Sn, Pb, and Zn. Metals were randomly present in the samples, and no significant differences could be observed among the three different polymers analyzed.
Ba was found in the highest concentration in the three polymers analyzed, exceeding the limit set by the European Pharmacopeia and the USP in 88% of the samples. Although not in elevated concentrations, Pb exceeded the limit set by the USP in 100% of the polymer samples. Zn was the only element that migrated into the glucose and NaCl solutions prepared as extraction media or as commercial formulations. Glucose solutions are more effective in promoting Zn extraction than the NaCl solution. Migration from PVC was higher than that from EVA and PP. The contribution of the administration sets in leaching Zn is noteworthy. Despite maintaining contact with the infused solution only during administration, the Zn content increased on average 52% after percolating the PVC set.
This study showed that, although being present in the assessed polymeric materials, the levels of leached metals were low and were unlikely to significantly contribute to the elemental impurity profile of the infusions therapy solutions.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgments
The authors are grateful to CNPq (Conselho Nacional de Desenvolvimento Tecnológico, Brazil) for financial support. The authors express their appreciation to the Hospital of the Federal University of Santa Maria for providing the samples used in this study.
- © PDA, Inc. 2019