Abstract
Adventitious contaminations with bacterial, viral, or fungal infectious agents represent a major risk associated with the manufacture and release of pharmaceutical products for human use, including vaccines, protein-based therapeutics, and antibodies. Early detection of contaminants in the biologicals production process might allow immediate action to correct such events without a significant interruption in the rate of production. Among the methods currently used for testing are cell culture, animal inoculation, electron microscopy, and in vitro molecular and antibody assays. Bacteria such as mycoplasma and mycobacterial species and most of the viral and fungal agents can take several days to weeks or even months to grow in culture. We have developed a broad-range microbial detection assay that uses the innovative Ibis biosensor platform, a rapid and high-throughput biosensor that is based on polymerase chain reaction (PCR) and electrospray ionization mass spectrometry to identify and quantify microbial contaminants. By combining the sensitivity of PCR with the accuracy of mass spectrometric detection, this technology generates a fingerprint that uniquely identifies an organism without a priori assumptions of the sample identity. This approach is capable of detecting known and unknown pathogens, as well as providing high-resolution genotyping and strain typing, and drug resistance and virulence information. Representative case studies are discussed here showing detection of minute virus of mice, mycoplasma, and an unknown virus that was identified as bluetongue virus.
- PCR
- Mass spectrometry
- Nucleic acid testing (NAT)
- Adventitious viral contaminant
- Mouse minute virus (MMV)
- Parvovirus
- Mycoplasma
- Bluetongue virus
Introduction
Adventitious contamination with bacterial (including mycoplasma), viral, or fungal infectious agents represents a major risk associated with the manufacture and release of pharmaceutical products for human use, including vaccines, protein-based therapeutics, and antibodies (1–3). Such contamination, be it unintentional or intentional (e.g., an act of bioterrorism), has deleterious effects, both financially and from the perspective of public health. Extensive and comprehensive testing for the presence of potential extraneous agents is therefore required to ensure the safety of these products. Early detection of contaminants in the biologicals production process might allow immediate decontamination of processing facilities without a significant interruption in the rate of production.
Currently, methods for pathogen detection and identification that can discover unknown adventitious as well as known microbial species in product samples are needed. In order to be actionable, these assays must provide sufficient species characterization information to allow corrective actions to be taken, while also providing clues to the source of contamination in order to avoid future events. Many of the current approaches require weeks or even months of observation and the time involved is often a constraint on product release. Among the methods used for testing are cell culture, animal inoculations, electron microscopy, and in vitro molecular and antibody assays. Some bacterial contaminants, such as mycoplasma and mycobacterial species, and most possible viral and fungal contaminants can take days to weeks or even months to grow in culture.
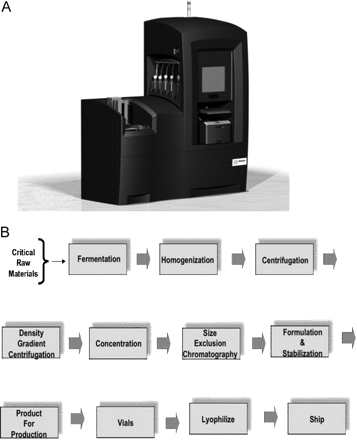
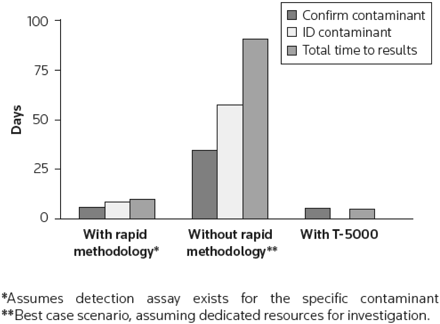
We plan to leverage the Ibis biosensor platform (Figure 1A), which uses mass spectrometry analysis of broad-range polymerase chain reaction (PCR) reactions for rapid, sensitive, cost-effective detection of adventitious agents, including previously unknown or uncharacterized organisms. The Ibis biosensor system, developed under a Defense Advanced Research Projects Agency (DARPA) program with support from the National Institute of Allergy and Infectious Diseases (NIAID), Centers for Disease Control (CDC), and the Department of Homeland Security, is a rapid, sensitive, high-throughput approach for detection and quantification of infectious organisms with applications in biological weapons detection, human clinical diagnostics, and human forensics. We propose to use this technology to monitor the pharmaceutical/vaccine production processes from beginning to end, reducing the lag time to results from weeks to less than 24 h. The system can be used to verify the sterility of processing environments and to detect mutations in the bioactive components. Due to the high-throughput, completely automated format of the assays on this platform, testing can be performed at many more stages and with larger numbers of samples. Potential uses in the pharmaceutical industry are listed in Figure 1B. An Ibis biosensor system currently in place at Amgen is being piloted as a research platform for monitoring biological product safety and for environmental monitoring. Some of the initial work has been described recently (1, 4). Figure 2 provides an evaluation of a hypothetical contamination event and detection and identification turnaround times using rapid methodologies. It is evident that a universal detection mechanism using a single-platform approach is most advantageous for the rapid response to a contamination event.
A) Ibis biosensor system. B) Bioprocess workflow. Adventitious agents may enter the process at any step.
Comparison of time saved using rapid detection methodologies. Figure taken from reference 1.
Methods
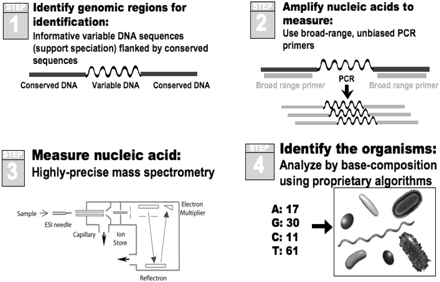
The Ibis biosensor couples broad amplification via the coupling of PCR with electrospray ionization mass spectrometry (ESI-MS) in a system known commercially as PLEX-ID (http://www.ibisbiosciences.com/). The basic principle of operation is illustrated in Figure 3. In brief, multiple pairs of primers are used to amplify carefully selected regions of the pathogen genomes. Following amplification, a fully automated ESI-MS analysis is performed. The mass spectrometer effectively weighs the amplicons, or mixtures of amplicons, with sufficient mass accuracy that the composition of A, G, C, and T can be deduced for each amplicon present. The base compositions are compared to a database of calculated base compositions derived from the sequences of known organisms to determine the identities of any microorganisms present. Thus, PCR/ESI-MS analysis provides detailed information analogous to that obtained using a microarray or parallel DNA sequencing instrument, but on a high-throughput platform that enables cost-effective analysis of large numbers of specimens. The mass spectrometer analyzes each PCR reaction in less than 1 min using no consumable products, and it operates in a completely automated fashion. The technology platform enables simultaneous identification and quantification of virtually all bacteria, all major groups of pathogenic fungi, and the major families of viruses (5–8).
Schematic of the PCR/ESI-MS approach for pathogen detection. 1) Regions are chosen from genomic alignments that allow amplification of highly informative segments of conserved genes from broad groupings of organisms. 2) Following nucleic acid isolation from the specimen, PCR amplifies short DNA fragments. 3) These amplicons are “weighed” with a mass spectrometer. 4) Mass is converted to base composition and this unique sequence information allows pathogen identification.
Broad Bacterial Identification
We have developed assays that analyze specimens on the PCR/ESI-MS platform using a panel of 16 broad-range primer pairs that have been developed to optimize unbiased coverage of all bacteria (refined from the set described in reference 7). Of the 16 primer pairs, four are targeted to the most conserved regions of the 16S rDNA, two targeted to the 23S rDNA, and the remaining 10 targeted to genes that encode universally conserved housekeeping genes. Use of six rDNA-targeted primers assures sufficient redundancy to avoid missing bacterial species imperfectly matched to one or two of the primer pairs. Primer pairs targeted to conserved housekeeping genes are not universal but are designed to amplify broad divisions of organisms (the precise coverage of this primer set is shown in Figure 2 of reference 8). This augments the rDNA primer pairs by further parsing the divisions of bacteria and increasing the dynamic range of detection limit when mixed populations of bacteria are present. Information from all 16 primer pair amplifications is used in aggregate in identification and quantification of each specimen.
The schema that we use provides for highly sensitive detection and accurate identification of organisms down to the theoretical stochastic limits of PCR (7, 9). The sensitivity is achieved by several factors. Firstly, the primer sequences have been iteratively optimized for sensitivity with maintenance of breadth of coverage. Secondly, PCR is carried out to 40 cycles and an end point measurement is made by highly sensitive mass spectrometry. Limiting dilution studies with carefully calibrated bacterial DNA standards have consistently demonstrated that PCR/ESI-MS achieves the theoretically maximum achievable sensitivity. Thirdly, broad-range priming is exquisitely sensitive to contaminating bacterial DNA from reagents. Contaminating bacterial DNA has been reduced to near zero by an industrial process of manufacturing material in a closed system where a final filtration step removes remnants of bacterial DNA. This was developed to manufacture materials for human clinical trials under a good manufacturing practices quality system with extensive quality control (QC). The only other possible contaminating DNA comes from the Taq polymerase; this contamination has been reduced to the lowest practical level, approximately 10 copies/PCR reaction, by QC manufacturing processes and lot testing. The base composition signature for each primer set of the E. coli strain used in the industrial fermentation of Taq is known and is readily identified as such in experiments.
Viral Identification
Adventitious viral contamination may be caused by a number of different viral species from diverse viral families or genera. These include RNA viruses (both positive and negative strand viruses), retroid viruses, and DNA viruses (single-stranded, ssDNA, and double-stranded, dsDNA) (1–3). Broad viral detection and identification is achieved by using target genes such as helicase or RNA-dependent RNA polymerase that are conserved within all members of a viral family or genus, and by combining several such primer pairs into an assay panel. Using this approach we previously demonstrated broad detection of respiratory viruses (5, 10, 11). Similarly, other viral panels target encephalitic viruses (12), bio-threat viruses, and blood-borne viruses, to name a few.
ESI-MS Analysis
The ESI-MS process separates the two strands of each PCR product, and both strands are used to corroborate the result and to properly assign base compositions for each detected PCR product. A discussion of the approach used in our detection software is available in Hofstadler et al., 2005 (9). In addition to external positive and negative controls, each assay includes an internal control (calibrant) that serves as an internal positive control for the PCR reactions and as a quantitative calibrant for the reactions. The calibrant is a DNA or RNA template that has the same primer-binding target sequences as the microbe, but the amplified region contains a deletion or insertion relative to all targets that causes a mass shift of ∼1500 Da or more per product strand. An approximate template copy number within a PCR reaction is quantified by comparing mass spectrometry signals obtained simultaneously for calibrant and target nucleic acid within the same PCR reaction. Because the calibrant and target have the same binding sites, the calibrant also competes with the target in the PCR reaction. Appropriate calibrant levels are determined experimentally to ensure the desired lower detection limit. This intra-reaction competition also serves as the basis for quantifying target input levels based upon signal output levels relative to calibrant signals. For a more detailed description of calibrated PCR using the Ibis ESI-MS system, see Hofstadler et al., 2005 (9).
Results and Discussion
Mycoplasma Detection
The Ibis biosensor system currently in place at Amgen is being piloted as a technology platform for assuring the safety of biological products. Mycoplasma-containing specimens were used to demonstrate the robustness of the biosensor in the detection of multiple organisms, as well as its comparability with standard nucleic acid–based methods (1, 4). These studies demonstrated that the Ibis biosensor was able to correctly identify each challenge species with a 1 day turnaround time. The commercially available sequencing-based method incorrectly identified three out of seven challenge species and had a turnaround time of 5 days. Additional testing was done using cell lines infected with mycoplasma species. Two different nucleic acid–based platforms (Ibis and Applied Biosytems, Inc.) were used to detect the mycoplasmas and showed detection in all but two dilutions tested. In contrast, results from both the standard 28-day culture assay and hybrid culture quantitative PCR assay were negative; no mycoplasma species were detected in these samples by these methods. More significantly, the Ibis system was able to identify that two distinct mycoplasma species were present in the NKm cell line: Mycoplasma fermentans and Mycoplasma hyorhinis, a result not easily achievable by standard sequencing techniques, which require a pure, homogeneous sample to obtain clean and interpretable sequencing data. Specificity testing using the Ibis biosensor system identified the actual bacteria that the system detected, thus providing a conclusive identification result that excludes mycoplasma. It also points to the value of Ibis technology to identify what, if anything, is contaminating a sample rather than simply determining if mycoplasma is present or absent. The Ibis biosensor system was able to correctly identify all single specimens. Mixtures of two and three mycoplasma species were also correctly identified. When mixtures were raised to four and five different species of mycoplasma, the Ibis missed one or two species from the mix.
Parvovirus Detection
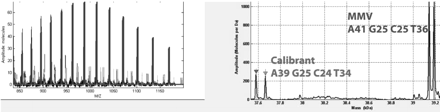
One of the assays developed on the Ibis platform provides broad coverage of all Parvovirinae. Parvovirinae represent a family of small ssDNA viruses that are ∼4–5 kb long. They can be divided into (i) Dependovirus genus that includes the human helper-dependent adeno-associated virus (AAV) serotypes 1 to 8, the autonomous avian parvoviruses, and the adeno-associated viruses (AAV 1–8); (ii) Erythrovirus genus that includes the bovine, chipmunk, and autonomous primate parvoviruses, including human viruses B19 and V9; (iii) Parvovirus genus that includes parvoviruses of other animals and rodents (except for chipmunks), carnivores, and pigs, including murine minute virus (MMV); and (iv) Bocavirus genus that includes the bovine and canine parvovirus and human bocavirus, which is a respiratory pathogen. Many of these parvoviruses can infect several cell types and have been described in clinical samples. AAVs in particular have been implicated in decreased replication, propagation, and growth of other viruses. In order to detect the presence of parvoviruses in cell lines, we developed a pan-parvovirus assay that broadly targets the various genera/species listed above. The primer pairs used in these assays targeted the NS1 and VP1 gene segments of parvoviruses. Samples containing various parvoviruses were obtained from ATCC and other collaborators and tested on the Ibis platform. An example of MMV detection is shown in Figure 4. Here, a blinded specimen containing MMV was tested on the parvovirus assay. Two primer pairs targeting the Parvovirus genus identified the sample as containing MMV. In addition, MMV detection was seen at a relative abundance of 5× greater than the synthetic control DNA, which was spiked into the sample at 100 copies per PCR reaction, suggesting the presence of MMV at about 500 genome equivalents of this virus. In other studies, the time course of MMV detection was studied, which showed MMV detection as early as day 2, comparable to specific real-time PCR assays (data not shown). Thus, the Ibis platform provides both sensitive and broad detection capabilities for parvoviruses.
Detection of MMV using a Parvovirus kit. The left panel shows a charge state distribution of the raw signal, whereas the right panel shows deconvolved spectrum. Blue indicates MMV detection and red indicates detection of the internal control (Calibrant) DNA. Relative abundance ratios between these two signals can be used to estimate the quantity of the target virus.
Unknown Virus Detection
An interesting case study describes the use of the Ibis biosensor technology for the identification of an unknown viral contaminant. This work was done in collaboration with Genentech and described previously (13). Genentech isolated a novel virus of unknown identity. The virus replicated rapidly (1–3 days) to a relatively high titer in many cell lines including monkey, human, and Chinese hamster ovary cells. Attempts to completely characterize this virus using existing methods were unsuccessful and based on the size and shape of the virus. While it resembled other positive-strand viruses, all attempts to identify a plus-stranded RNA virus failed. The Ibis system was used in an attempt to broadly identify the family (or genus) to which the unknown virus belonged. Eighty different primer pairs capable of detecting more than 40 genera and hundreds of species of DNA and RNA viruses were used for pan-viral screening. Four different total RNA extraction samples were tested, including RNA from non-infected and virus-infected Vero cells, and RNA from sucrose-cushion-purified and CsCl-purified virus. A sample with no cell background was used as negative control.
Results from the pan-viral testing showed no detection of virus in any of the four samples in all but four primer pairs (data not shown). The four primer pairs, VIR3507-10, detected virus in samples 2–4, which were obtained from virus-infected cell line or purified viruses (Table I). In the control samples, with or without a cell-line background, no virus was detected. These four primer pairs were specifically targeted to the bluetongue virus (BTV) species, members of the Orbivirus genus from the Reoviridae family of viruses. BTV was subsequently confirmed as the correct virus based on work by Dr. Potts suggesting BTV as the putative adventitious “mystery virus” in the infected cell line.
Detection of BTV-Like Signature in the Four Infected Cell Lines
Base composition detections obtained using the Ibis T5000 provide detailed fingerprint signatures and enable further characterization of the virus species. The four primer pairs used for BTV detection in this study were all targeted to the BTV L1 segment (3944 bp) that encodes the capsid VP-1 gene. All four primer pairs produced base compositions that were compared to genomic signatures of existing BTV genomes from Genbank. These results are shown in Table II. Genentech BTV matched BTV serotype 10 and BTV 17 in three of the four primer pairs tested, but was different in primer pair 3507 by one or two single-nucleotide polymorphisms, respectively. Thus, GEN-BTV probably represents a novel bluetongue variant.
Base Composition Signature Analysis of BTV Amplicons in Genbank. Genentech BTV (GEN-BTV)
Conclusions
The Ibis biosensor system provides a single, high-throughput platform for detection, quantitation, and identification of microbes, including simultaneous identification of multiple species in a single sample. Thus, it significantly reduces the time to results, which can be advantageous for formulating a rapid response and root-cause analysis of a contamination. The presence of internal calibrants allows for the relative quantification of genome copy numbers in the sample being analyzed. The ability of the Ibis biosensor to provide results in a fraction of the time it currently takes highlights the limitations of traditional methods in the investigation of contamination events in the biopharmaceutical industry. The Genentech BTV case study highlights the ability this platform to detect a novel variant of a BTV species for which sequence data was not available, demonstrating the utility of the technology in detecting and characterizing previously unknown viruses.
Acknowledgments
The authors acknowledge funding from NIAID under contract number HHSN266200400100C/NO1-AI-40100. Some of the samples results reported here were based on samples provided by Dr. Houman Dehghani, Bill Lawrence (Amgen), and Dr. Barbara Potts (Genentech).
- © PDA, Inc. 2010