Abstract
Isolators provide a high degree of protection for the product and/or the environment and operators in pharmaceutical production, as well as for analytical and sterility testing. Gloves allow for performing testing and for easy access to the process. Due to their nature—thin plastic, highly flexible—and their risk of puncture or rupture, they are regarded as one of the main potential sources of contamination. Glove integrity testing is therefore a main issue and has been addressed by many regulations such as those imposed by the USP, U.S. Food and Drug Administration, and Pharmaceutical Inspection Convention.
This paper presents a short overview of different glove integrity test procedures and their ability to detect leaking gloves. Additionally, extensive microbiological tests have been performed to give more evidence and cross-correlation to physical testing. Most of the physical tests have limitations either in detecting pinholes and/or they are difficult to implement for routine testing. Microbiological tests are only applicable for evaluation and validation purposes, but not for routine testing, because they are time-consuming and do not allow immediate action. Routine visual verification of gloves by trained personnel turns out to be a very reliable technique.
Additional microbiological tests supported by microbiological environmental monitoring helped to develop a new concept presented here on how to handle gloves with pinholes. It is proposed not to automatically consider a pinhole in a glove as a breach in isolator integrity, but to consider any action in view of controlling and monitoring the effective bioload on the outside of the gloves. With the combination of semi-automatic physical testing with independent protocol, visual inspection, and control of bioload through microbiological environmental monitoring potential contamination, risks can be minimized and maximum safety maintained.
LAY ABSTRACT: Isolators are enclosure designs to protect critical handling and process steps in pharmaceutical environments. They provide a high degree of protection for product and/or environment and operators against particles, potentially hazardous active principles, and microbial load.
Gloves mounted on windows and doors of the isolator allow for manipulation, performing testing, and access to the process. Due to their nature and their use with risk of puncture or rupture, they are regarded as a potential source for contamination. Glove integrity testing has therefor been addressed by regulations such as those imposed by the USP and the Food and Drug Administration.
This paper presents a short overview of various glove integrity test procedures and their ability to detect leaking gloves. Most of the tests have limitations either in detecting pinholes and/or they are difficult to implement for routine testing. Routine visual verification of gloves by trained personnel turns out to be a very reliable technique.
Additional microbiological tests led to a new concept presented here on how to handle gloves with pinholes and how to take action. With this approach, risks can be minimized and maximum safety maintained by controlling and monitoring the effective bioload on the outside of the gloves.
Introduction
Gloves To Isolate Process
Isolators for pharmaceutical applications are typically placed in rooms that are either classified as class 100,000 in operation (corresponding to grade D or ISO8) or, for sterility testing isolators, are non-classified. For aseptic processes, the isolator volume is generally decontaminated using hydrogen peroxide or another sporicidal agent applied in a sprayed, vaporised, or gaseous form. Gloves are used as a tight but flexible interface for process intervention or performing sterility or other analytical testing. In order to fully maintain the integrity of the whole system, the process, and the protection of the personnel and environment, it is thus indispensible to check glove integrity routinely to avoid contamination on both sides of the interfacing plastic glove.
Gloves are often used with sleeves in order to improve reaching area of the hands. Therefore, the entire glove-sleeve assembly represents a risk for a breach in isolator integrity and thus a potential route of contamination. It is our experience that approximately 80% of defects are related to gloves but only 20% to sleeves. Moreover, sleeve defects are very often due to improper hook-up (e.g., incising brackets) or inappropriate position of the glove port or work material (e.g., stretching upon use of the gloves). These causes can be fairly easy recognized and overcome. Contrary to gloves, sleeves exhibit less risk for potential contamination due to minimal direct contact with objects, tools, and instruments. As a consequence, this study focuses on gloves and not on glove-sleeve assemblies. All of the tests presented were made with gloves only.
Gloves provide smooth handling for operations with ampoules, needles, syringes, vials, tubing, filling needles, and for cleaning. This makes them become a critical weak point. Glove thickness is mostly well below 1 mm. Contact with sharp edges, for example, from debris, needles, or scissors, and the continuous flexing and exposure to chemicals such as hydrogen peroxide, involve a major risk of defects.
This risk has been well recognized, and verification of glove integrity has become routine. It also has led to the development of integrity test equipment and procedures for glove testing. Routine glove integrity testing is mandatory as per many regulations. “Very small leaks in gloves are difficult to detect until the glove is stretched during use. There are several commercially available glove leak detectors; the operator ensures that the detectors test the glove under conditions as close as possible to actual use conditions. Microbiological tests are used to supplement or substitute physical tests,” according to USP 32 〈1208〉 Validation of Isolator Systems, (1). Similar requirements are stated by the Pharmaceutical Inspection Convention (PIC/S) Recommendations; Isolators Used for Aseptic Processing (2). The Food and Drug Administration (FDA) Sterile Drug Products Produced by Aseptic Processing Guide (3) indicates that “a faulty glove or sleeve (gauntlet) assembly represents a route of contamination and a critical breach of isolator integrity. With any use gloves should be visually evaluated for any macroscopic physical defect. Physical integrity tests should be performed routinely. The monitoring and maintenance program should identify and eliminate any gloves lacking integrity.” This implies the need for training programs to successfully identify pinholes in gloves as well as including gloves in routine microbiological environmental monitoring programs.
Practice confirms that pinholes in gloves may be difficult to be detected. Furthermore, the risk of contamination by micro-organisms that migrate through pinholes in gloves is not well quantified. In view of this uncertainty and the importance of the proper barrier function of gloves in isolator technology, this study has been conducted
-
to evaluate the performance of various glove integrity testing methods
-
to quantify realistic bioloads on the outside of gloves during production or sterility testing
-
to evaluate the potential risks of microbial contamination and migration through glove pinholes and therefore quantify the potential of a product or test contamination risk
-
to develop criteria and strategies on how to handle pinholes in gloves and risks associated therewith.
The main focus of this study was to quantify the relevance of pinholes to potential risks of contamination of work or product processed in isolators. Thus, microbiological aspects and the knowledge of factors influencing risks associated with pinholes are in the foreground. Finally this understanding leads to well balanced concepts and strategies to reduce these risks efficiently.
This study has been conducted in common effort and close cooperation between an isolator manufacturer, a university, and a pharmaceutical company (4).
Materials and Methods
Preparation of Pinholes in Gloves
When checking the integrity of gloves, one will recognize that pinholes as perforations very seldom occur, but clefts are typically found in most cases. As clefts are more difficult to detect, they are considered more critical and were therefore chosen for this study. (For practical reasons, the term pinhole is kept as standard throughout this study and indicates any kind of defect in gloves.) In order to undertake the testing of gloves in a most reproducible and standardized manner, it has been essential to first prepare gloves with reproducible and standardized pinholes of defined size.
All of the gloves tested in this study were made of Hypalon®, a chlorosulfonated polyethylene, white in appearance. They are very smooth and give high comfort in use. Their chemical resistance is excellent, as well as their mechanical properties including their characteristics regarding heat and flames. The sizes tested (sizes 5–8) have a typical thickness of about 0.4 mm. Usually they are used over a period of a few days up to several months depending on integrity.
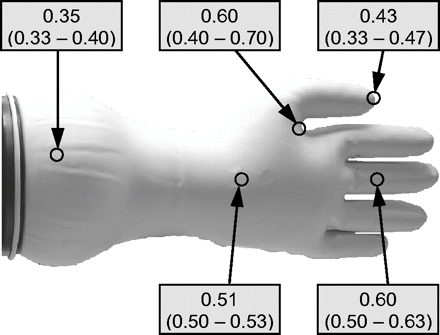
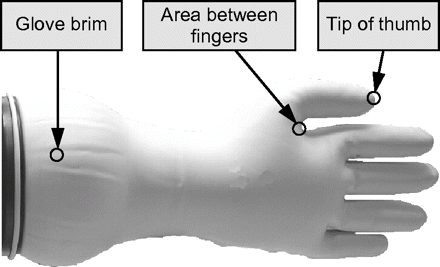
First, three gloves of each of different sizes 5 to 8 were tested for thickness (using a sliding rule) at 5 specific areas: fingertip, finger sleeve, area between thumb and forefinger, inner hand surface, and glove brim. Results show that thickness is in the range of 0.3–0.7 mm (specified value: 0.4 mm) and that the fingertips and the glove brim have less thickness than other parts (Figure 1). No correlation was found between glove size and thickness. Known from years of practice, fingertips and the area between thumb and forefinger are most sensitive to loss of integrity. Based on these facts the following leak positions were selected for the integrity test experiments given below (Figure 2):
-
tip of thumb (position F): strong product contact probability, difficult to detect
-
area between thumb and forefinger (position S): strong stretching during use
-
glove brim (position E): critical contact position with glove pulled back.
Measurement of material thickness (mm) with sliding rule.
Definition of leak positions.
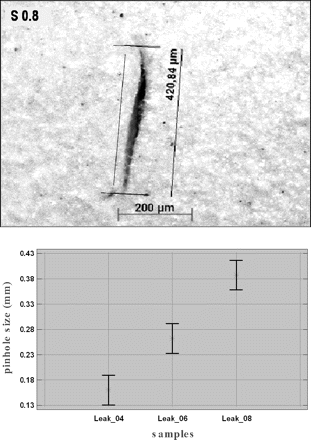
In order to create pinholes in a reproducible manner, the gloves were punctured with injection needles having external diameters of 0.4, 0.6, and 0.8 mm. The pinholes created are like small slits (clefts) and were recorded with a digital camera using microscopy and standard software to size the cleft width (see Figures 3a and 3b).
(a) Magnification of pinhole S 08. (b) Pinhole size (mm) as function of needle size (means and 95% LSD intervals).
Mean values of cleft width found were
-
0.16 mm (0.4 mm needle size)
-
0.258 mm (0.6 mm needle size)
-
0.387 mm (0.8 mm needle size)
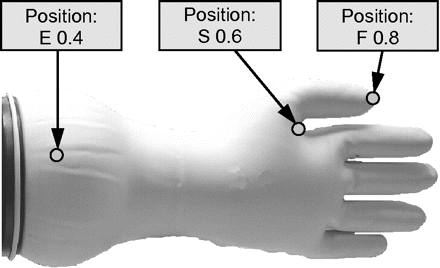
A summary of the statistics of this test is shown in Table I. This way of preparing pinholes delivers statistically significant different leak sizes (see Figure 3b). Reproducibility and standardization are thus assured. For all physical and microbiological tests, glove pinholes were made accordingly and denoted with position and size (e.g., S 0.6, F 0.8 or E 0.4) as illustrated in Figure 4. For all the following tests at least three sets of test gloves of each leak position and leak size where prepared and used to evaluate potential variances caused by the test glove preparation. All of these gloves were of the same glove size (size 8).
Summary Statistic of Pinhole Size after Perforation with Three Different Needle Sizes
Definition of leak position and size.
Physical Glove Integrity Test Methods
It was not the intention of this study to extensively test equipment for physical glove integrity testing. All of this testing has been performed with only one glove material (Hypalon) and glove size (no. 8) from one manufacturer. Thus the findings are limited and may not be generalized for other materials, other manufacturers, and so on. For this reason the results are given only as an indication.
Six physical methods were investigated and compared for the following criteria:
-
capability for reliable detection of pinhole(s)
-
quantitative (proportional to leak size) or qualitative (just “YES/NO”) leak detection
-
selective (leaks can be localized and identified) or cumulative (overall leaks) detection of leak position
-
suitability of the method for routine use, either prior to or during production/test
The methods are shortly presented here with their principle and major features. Table II summarizes the findings for comparison. On the whole it confirms the statement of the USP on the limited capacity and difficulty to reliably detect small leaks with physical methods.
Comparison of Features of Physical Glove Integrity Test Methods
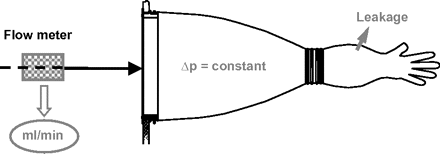
1. Flow Test
An air pressure of about 600 to 1800 Pa is used to blow up the glove and is then held to a constant value. Leakage flow through the glove is measured by means of a sensitive flow meter (per glove) over a period of 5 to 60 min (Figure 5). Flow values obtained above a set limit value are considered to indicate a pinhole. Higher test pressures enhance detection of pinholes. Main advantages of this method are non-destructive and non-contaminating, applicable from outside the isolator, easily automated and with record protocol, multiple glove testing, and in-place testing during downtime periods. Still there are some drawbacks: large differences in values obtained upon type, material and size of gloves, and sensitivity to pressure build-up history and to temperature changes. Still, the leak position is not localized.
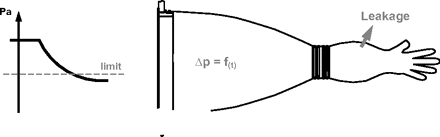
2. Pressure Drop Test
The pressure drop test consists of virtually the same principle as the flow test, with the only difference to monitor the pressure decay (instead of the flow) as an indicator of a leaky glove (Figure 6). Higher test pressures enhance detection of pinholes. Advantages and disadvantages are therefor the same as for the flow test.
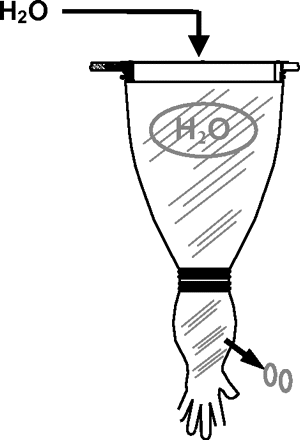
3. Water Breakthrough Test
The water breakthrough test is similar to the flow test, but utilizes water pressure to inflate the glove. Visual inspection of the glove to recognize penetrated water droplets allows the localization of a leak (Figure 7). Whereas reproducibility, detection capability, and sensitivity are good and localization of a hole is well possible, the suitability for practice and routine use is not given: the test can only be performed with hanging gloves, water volumes exceed easily 1 L for the glove (and several liters including the arm sleeves), and the wet glove surface may enhance microbial growth/migration and further contamination. The test cannot be applied during routine use.
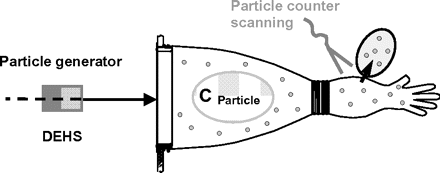
4. Particle Penetrating Test
In the particle penetrating test, a high-particle or aerosol (e.g., diethylhexyl-sebacate) concentration is applied to challenge one side of the glove. By scanning the other surface side with a particle counter probe, leaks can be detected by raised particle counts around these areas (Figure 8). Sensitivity and detection capability are lower than for the water breakthrough test. It suffers most of the disadvantages of that test too: contamination of the glove by particles, complex test setup, time-consuming scanning, not automated, and not suitable for routine use or production.
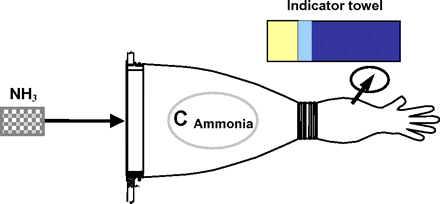
5. Diffusional Test (with Different Types of Chemicals Including Helium, Ammonia, Peracetic Acid)
Diffusional tests using chemicals have been widely applied for leak/tightness testing (e.g., flexible film isolators). The principle is the same as for the flow test with the difference that leaks are detected by specific chemical indicator probes instead of flow or pressure monitoring (Figure 9). Whereas helium is “clean” and non-contaminating and scanning can be done with an electronic probe, sensitivity and practicability are fairly poor, and spurious noise signal may interfere. Both peracetic acid and ammonia give better results in these respects but do contaminate the gloves (and more), have very strong odor, and need special pH indicator towels for wiping the gloves to detect leaks. Positive localization of leaks is good and fast, but the method is not really suitable for routine or in-production testing.
6. Visual Inspection
Visual inspection does not need specific equipment but is a time-consuming procedure. Evaluation of the method led to the clear result that untrained personnel will detect only about one third of leaks. However, trained operators were able to identify 99 of 100 leaks. This is as good as with the water breakthrough test but without the drawbacks. Factors affecting the detection rate include good visibility, glove material and surface properties, color of gloves, and personnel skill. Also, it is required to access the gloves from the work space. Thus, the inspection can only be performed at the isolator when opened. A main advantage of the visual inspection is that pinholes in fingertips are more easily recognized than with automated flow test equipment.
Flow test principle.
Pressure drop test principle.
Water breakthrough test principle.
Particle penetrating test principle.
Diffusional test principle.
Summary of Results
Table II shows the results of the various test methods and their features in accordance with the criteria posted.
Discussion of Results and Conclusions Regarding Physical Testing
All of the instrumental integrity test methods showed clear limitations to detect pinholes, especially those created with the 0.4 needle and at finger tip position (F). In many cases, a differentiation between the sizes 0.4 and 0.6 mm was not possible. All of the diffusional tests with helium, peracetic acid, or other chemicals, as well as the particle test, have only limited detection capabilities. Furthermore, their implementation as a routine control is difficult and imports other risks of contamination, for example, particles or residuals of the chemicals. This is true even for the water breakthrough test, although it has shown very good detection rates. However, for the risks cited above, it is not considered as a suitable routine test for gloves.
The flow test and its twin pressure drop test have both been more easily adopted in daily use and can well be applied to gloves finally installed. The detection rate is highly dependent on test parameters such as pressure applied and limit value. Pressure build-up and history of pressurizing have an impact. Results are more sensitive and consistent with gloves that have been pressurized before testing (e.g., pressurized to 2000 Pa for 5 min as preconditioning, then tested at 1500 Pa for integrity). Both tests can be performed with (semi) automated equipment and allow multiple parallel testing. Testing under production conditions is possible for the flow test by applying a negative test pressure. They suffer the same drawbacks: to obtain reliable results, the temperature of the whole setup (instrument, isolator, and environment) must be thoroughly stable and there should be no atmospheric pressure changes during testing. To demonstrate susceptibility to temperature changes one can apply the following rule of thumb: 1 °C change in temperature results in a pressure change of about 300 Pa (for a fixed volume). Thus, if verifying limit values based on a difference of only 100 Pa lower pressure in the pressure hold test, one must be aware that temperature changes of −0.3 °C may account for 100% of that allowable pressure drop, theoretically. Long test durations are disadvantageous due to these effects. In practice, the dependency on temperature is found to be less dramatic because of the flexible nature of the gloves, which, however, covers part of the changes that would not be the case for any other 100% rigid volumes. Concurrently this effect also reduces the sensitivity of the test itself and accounts for background signal shifts influencing the result.
Type and material of the gloves are sources of variabilities observed of diffusional volume flow and have an important impact on test results. Test conditions as evaluated for a specific type/material of glove cannot be transferred to another type/material without preliminary analysis.
Visual testing turns out to be very effective. With well trained operators, the recognition rate approaches 100% (Table II), especially for pinholes at fingertips, which are better detected than with physical instruments. Thus we highly recommend performing routine tests visually as part of the job at the isolator. Sensitivity and reliability are strongly correlated to the operator's tenacity when performing the inspection. Training and maintaining this skill is thus imperative.
Microbiological Tests: Growth Through Pinholes
The FDA addresses risk associated with missing integrity of isolators systems with emphasis on the need to control not only glove integrity, but also the sanitary quality inside gloves: “Due to the potential for microbial migration to microscopic holes in gloves and the lack of highly sensitive glove integrity tests, we recommend affording attention to the sanitary quality of the inner surface of the installed glove and to integrate the use of a second pair of gloves” (3). Control of bioload and microbiological environmental monitoring of gloves are thus mandatory.
The first set of growth-through tests has been performed on the basis of sterilizing filter tests: the glove inside is challenged with a highly concentrated bacterial suspension; the exterior of the glove is covered with nutrient media. Pinholes will be detected as turbidity in the nutrient media caused by growth through pinholes.
Test Setup for Growth-Through
A suspension of Brevundimonas diminuta (a Gram-negative roll shaped bacteria of small size, as used for sterilizing filter validation studies) has been prepared in nutrient media (tryptic soy broth, TSB) to result a challenging concentration of 1.6 × 108/mL of micro-organisms. Presterilized gloves, three having full integrity and 27 with defined pinholes (three each with pinholes at three leak positions and from three needle sizes), one per beaker, are immersed into 1700 mL of a sterile nutrient solution (tryptic soy broth, TSB) in a 3000 mL beaker glass and the brim of the glove used as flexible closure of the beaker as illustrated in Figure 10. Upon that, 1000 mL of the B. diminuta suspension is poured into the glove inside. After covering the beakers, these are put in the incubator and visually checked for growth-through after 2, 7, and 14 days of incubation at 30–35 °C.
Growth-through test principle.
Test preparation:
-
Test organism: Brevundimonas diminuta
-
Concentration: 1.6 × 108 colony-forming units per milliliter (CFU/mL)
-
Incubation temperature: 30–35 °C
-
Incubation time: 14 days
-
Growth evaluation: after 2, 7, 14 days
Results of Growth-Through
After 2 days incubation, already 24 out of 27 gloves with pinholes were detected by turbidity of the formerly clear nutrient media caused by growth-through of the test organism B. diminuta (not specifically identified). After 7 days incubation, 26 were positive, and finally all pinholed gloves were identified visually by growth-through after 14 days incubation. Parallel reference tests with integer gloves showed all negative after 14 days, confirming valid test conditions.
The microbiological tests for growth-through have demonstrated that micro-organisms do successfully migrate through pinholes of gloves. One hundred percent of the 27 defective gloves were detected. As a consequence: Does a production lot (production) or a test result (growth upon sterility testing) have to be rejected in either case when a glove with a pinhole has been found? What is the relevance of a defective glove to product quality, to isolator integrity, or to validity of a sterility test result in practice? What is the probability for microbial migration and thus contamination in practice?
Results up to this point led to the conclusion that any measure taken shall be based on a risk assessment that investigates real risk potential for the condition defective glove in practice. In order to answer the above questions and comply with the idea of a risk assessment, a new set of tests has been conducted as shown hereinafter.
Microbiological Tests and Simulations with Realistic Bioloads
Bioload of Gloves in Routine Use:
The microbiological tests described previously are not very realistic. In assessing risks as to contamination of the isolator environment or the product through defective gloves, there remain questions as to the potential bioload on the inner surface of the gloves when working at the isolator and as to the chance of micro-organisms migration through pinholes in gloves, effectively contaminating the product side.
In order to make a valid selection of realistic bioload, a large number of gloves from various isolators (from production and sterility testing) were tested for their current bioload on the inner side of the glove. Samples were taken with contact plates 25 cm2 and analyzed accordingly. Furthermore, the gloves were inversed and disinfected with 70% isopropanol and samples newly taken at a minimum 1 h to maximum 12 h later to indicate any change in bioload over time.
The results confirm a typically very low bioload of the gloves' inner side under routine use (see Table III): from 103 gloves, 30 showed no growth when tested for current bioload, with a maximum of 41 CFU per sample. One to 12 h after disinfection of the inner side of the gloves, 89 out of 103 showed 0 CFU, with a maximum bioload of only 4 CFU. Even after 11 production batches, which represents a 2 week period, less than 20% of the gloves showed more than 5 CFU/sample.
Bioload on Glove Inner Side; Samples Taken from Existing Production Systems (“After Disinfection” = 1–12 h After Applying Disinfection)
Based on these facts, the following tests have been more practically leveled and will use low to medium level bioloads in contrast to the previously described growth-through test.
Preparation of Gloves with Realistic Bioloads:
A suspension containing a set of micro-organisms collected from the skin of the experimenter (called here human skin flora; the micro-organisms within this set have not been identified) as used in the subsequent growth-through tests, has been created to provide a synthetic standard suspension pool. From this, dilutions were prepared with 108 CFU/mL down to 102 CFU/mL. Gloves were inversed (inner side to outside) and then dipped into the various dilutions to wet the inner surface of the glove to build up the challenging bioload. The gloves were then dried in air for 5 min. The original glove outside surface was disinfected with 70% ethanol so as not to falsify the testing. Then 1 cm2 pieces were cut out of the gloves and placed in 10 mL of phosphate buffered peptone saline solution, (PBPS), to extract the micro-organisms from the glove. In order to get an estimate of the reduction of micro-organisms over time, another 1 cm2 sample was taken just after drying, after 2, 4, and 6 h and poured in 10 mL of PBPS. Samples of the extract were then placed on sterile petri dishes, covered, and mixed with tryptic soy agar, placed in the incubator at 30–35 °C for 48 h, and then counted.
The results of this microbiological test are shown in Table IV. Three major effects can be seen:
Glove Contamination Test
-
Bioload found on the test samples was proportional to the challenging suspension.
-
Over time (2–6 hours after application) the bioload decays to much lower values.
-
For low bioloads at t = 0 this decay reaches quickly 0 CFU/sample after 2–6 h.
Conclusions from Real and Artificially Prepared Glove Bioload:
Three major conclusions can be drawn:
-
Micro-organisms on gloves do not tend to grow over time but to die off. Initial concentrations tend to go down, as shown in Table IV.
-
Simple disinfection of the hands and of the gloves' inner side with 70% isopropanol effectively reduces the bioload to very low CFU counts (see Table III) and thus further reduces the chance of micro-organism migration even in case of pinholes by lower challenge levels.
-
The effective bioload as found on the inner surface of gloves in use is low, with typically 3–10 CFU/cm2 (derived from Table III), by far lower than the challenge concentrations prepared (773 CFU/cm2 after 2 h).
As the microbiological process simulation tests to be described below will run over a period of 2 h, the concentrations found after 2 h are considered to be the representative concentrations. The tests have been conducted with the two highest concentrations (made from 1 × 108 and 1 × 107 CFU/mL suspension, respectively) as well as with the most realistic concentration (made from suspension 1 × 104 CFU/mL suspension) (Table V).
Glove Test Contaminations Selected for Microbiological Process Simulation Tests
Practical Process Simulation Tests:
This test setup shall demonstrate the real migration of micro-organisms from the inner glove side of correctly installed and previously decontaminated defective gloves onto product or the environment of isolators. Product in this case means glass marbles of 15 mm diameter that are manipulated in a simulated work process. Additionally environmental monitoring shall reveal any contamination inside the isolator (see Figure 11).
Four gloves of a set were all punctured the same way with three needle sizes and at the three previously described positions and then mounted on a sterility testing isolator (total 36 defective gloves) and compared to one set of four gloves with full integrity. All material needed is placed inside the isolator, which is then decontaminated the standard way with vaporized hydrogen peroxide. The glove inner side is then contaminated as described above with different concentrations of micro-organisms (as described above) to build up defined bioloads: high, medium, and realistic. Now, an operator (using gloves 1 and 2) manipulates the 20 glass marbles: these are picked up with one hand, given over to the other hand and are deposited on a plate, repeating this transfer back and forth during 1 hour. This is then repeated with the other two gloves (gloves 3 and 4) for another hour, and the whole procedure repeated 4 more days. Each day the gloves are “bioloaded” with the appropriate micro-organism suspension again to maintain the contamination level. After each day four glass marbles are placed in nutrient media to determine transferred micro-organism counts (two glass marbles in TSB, for the detection of aerobic bacteria, yeast and mould, and the two other glass marbles in fluid thioglycollate media (FTM) for the detection of anaerobic and aerobic bacteria). Microbiological environmental samples recovered at this time include settling plates nearby the “transfer play area” and contact plates to check specific positions of the isolator and of the glove sleeves as well as all fingertips of the gloves, representing a routine microbiological environmental sampling of an isolator system. The tests are also conducted with gloves with full integrity and worst case bioload high of 3.6 × 104 CFU/cm2 as a reference.
Sterility test isolator as used for the practical process simulation tests.
Results from the Practical Process Simulation Tests:
The reference test with gloves with full integrity and worst-case bioload high showed no contamination. The results of the tests with defective gloves reveal that high bioloads and/or large pinholes may occasionally lead to contaminations either on the glass marbles or the environment (an example of such test results is shown in Table VIa and Table VIb).
Practical Process Simulation Test 1 Using High Bioload at Leak Position F 0.4 (CFU per Sampling Plate 25 cm2; “pos.” = Growth)
Practical Process Simulation Test “2” Using High Bioload at Leak Position F 0.4 (CFU per Sampling Plate 25 cm2)
Non-reproducible results as shown in Table VIa and VIb are typical for the test using high bioload (3.6 × 104 CFU/cm2): While one test occasionally shows contamination, a second set of the identical test did not show any contamination at all. Lower bioloads such as medium (4.3 × 103 CFU/cm2) or realistic (50 CFU/cm2) do not cause any contamination in spite of defective gloves. Table VII summarizes the findings where each combination of bioload, leak position, and leak size represents at least 1 week of testing.
Summary of the Practical Process Simulation Tests
Checking counts on the contact plates suggests that only sporadic contamination can be expected to be transferred onto product, but only for high, and thus non-realistic, bioloads inside the gloves. It was interesting to see the results of additional swab tests performed at the known gloves pinhole areas in addition to contact plate counts at predefined positions, representing a routine microbiological environmental sampling inside the isolator system (see Table VIIIa and Table VIIIb).
Contamination Found on the Glass Marbles (neg. = No Growth) and Inside the Isolator System as CFU/Sampling Plate 25 cm2 by the Predefined Microbiological Environmental Sampling program, S 04, High Bioload
Contamination Found by Swab Tests at the Known Leak Position (CFU per Sampling Plate 25 cm2)
When comparing results from the product (glass marbles) contamination and the microbiological environmental sampling program, (shown in Table VIIIa) to swab counts at known leak position (shown in Table VIIIb), it is evident that swabbing at the known pinhole area reveals much higher counts, especially at the area between fingers and at the brim. Apparently, in spite of detectable micro-organisms (swab count at leak position), no micro-organisms were transferred onto product (glass marbles) or the isolator environment. There is no correlation found between product contamination, environmental contamination, and leak point contamination. Pinholes are therefore not necessarily detected during routine microbiological environmental sampling.
Conclusion
Summarizing the extensive glove tests, the following findings are essential for physical glove testing:
-
All suitable physical test methods have limitations in detecting pinholes in gloves.
-
Typically, detection of pinholes at fingertips is weak.
-
Visual tests allow the detection of almost all leaks and therefore overcome instrumental limits provided that operators are well trained.
In respect to microbiological tests and risks of contamination by defective gloves, the tests confirmed the following:
-
Tight gloves are an effective barrier for microbiological contaminations.
-
Micro-organisms may successfully migrate through glove pinholes and therefore raise the risk of potential contaminations.
-
High bioload on the inner side of defective gloves does represent a contamination risk for product as well as for the isolator interior.
-
Bioloads found on gloves in practice typically show low concentrations of <20 CFU/cm2.
-
Medium to low bioloads, especially those at realistic levels, do not represent a contamination risk in combination with the defined leak sizes and positions.
There is unanimous consensus for the need of testing gloves. But the strict view that pinhole in glove leads to breach in isolator integrity leads to product or test potentially affected to be rejected or repeated does not consider real world situations and may also have enormous economic consequences. Based on the extensive test results presented here, a more rational view is proposed that supports visual testing, automated physical testing, and microbiological environmental monitoring by assessing the risk of contamination by defective gloves. From this risk assessment, all measures shall contribute to reliably identify leaks and to reduce risk of contaminations, and by doing so to minimize an effective breach in isolator integrity. The tests shown have demonstrated that even defective gloves will not contaminate a product if proper control of bioburden of the glove inner side and properly evaluated techniques are respected. Gloves which passed diffusional flow testing successfully do not represent a microbiological risk even in presence of a minor pinhole and a realistic bioload.
In order to comply with the high requirements of aseptic handling in isolators, the following set of measures is proposed to reduce the risk of contamination at best:
-
routine visual inspection (pinholes at fingertips are best identified with this method)
-
training and qualifying operators for visual inspections (essential, and keeps detection rates high)
-
routine use of automated flow testing equipment with protocol record as an independent method and tests conducted possibly even during production or testing in isolators
-
control of bioload by disinfection of hands and glove's inner side as well as microbiological environmental monitoring
-
use of redundant gloves, disinfected as well, as recommended by the FDA
Based on careful risk assessment and extensive evaluation, we consider this set of measures to effectively master the potential risk of contamination in the case of glove fracture. The development of even more sensitive and faster test equipment may further improve recognition of defective gloves. Improvement is also possible in the development of more perfect gloves.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2011