Abstract
The application of a head space analyzer for oxygen concentration was examined to develop a novel ampoule leak test method. Studies using ampoules filled with ethanol-based solution and with nitrogen in the headspace demonstrated that the head space analysis (HSA) method showed sufficient sensitivity in detecting an ampoule crack. The proposed method is the use of HSA in conjunction with the pretreatment of an overpressurising process known as bombing to facilitate the oxygen flow through the crack in the ampoule. The method was examined in comparative studies with a conventional dye ingress method, and the results showed that the HSA method exhibits sensitivity superior to the dye method. The results indicate that the HSA method in combination with the bombing treatment provides potential application as a leak test for the detection of container defects not only for ampoule products with ethanol-based solutions, but also for testing lyophilized products in vials with nitrogen in the head space.
LAY ABSTRACT: The application of a head space analyzer for oxygen concentration was examined to develop a novel ampoule leak test method. The proposed method is the use of head space analysis (HSA) in conjunction with the pretreatment of an overpressurising process known as bombing to facilitate oxygen flow through the crack in the ampoule for use in routine production. The result of the comparative study with a conventional dye leak test method indicates that the HSA method in combination with the bombing treatment can be used as a leak test method, enabling detection of container defects.
1. Introduction
In the manufacturing and shipping of sterile products such as parenteral injections, the prevention of microbial contaminations is a prerequisite. According to “Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing (2004)” (1), failures in detecting fractured ampoule products resulting from faulty machinery as well as from inappropriate handling of finished bulk stock have resulted in consequences such as drug recalls, suggesting the need to establish a detection system with sufficient accuracy for the bulk product to screen out container/closure defects. Annex 1 in the European Union (EU) guideline for good manufacturing practices (GMP) “Manufacture of Sterile Medicinal Products” (2) states that containers closed by fusion, for example, glass or plastic ampoules, should be subject to 100% integrity testing. The Japanese guideline, “Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing” (3), also states that the container integrity of the sterile drug products should be assured by conducting routine monitoring, in-process control test(s), and/or a 100% inspection of the containers for integrity during transportation and storage.
Several container closure testing methods are commonly employed, including the high-voltage leak detector method (4), the vacuum decay method (5), and the dye ingress method in commercial production (6). The high-voltage leak detector method, applicable to conductive product, is based on the detection of a small amount of electrical current generated on applying a voltage to ampoule products. For the vacuum decay method, applicable to both ampoule and vial products, a sample of interest is initially set in a closed container of equipment and subsequently exposed to reduced pressure for monitoring of the pressure changes (5⇓–7). The dye ingress method, on the other hand, is based on the visual inspection of a sample post-immersion in a dye-containing solution and is applicable only to ampoule products. This method tends not to be used to vial products as the complete removal of the dye between the cap and rubber stopper is often difficult. Injectable drug products may be largely divided into two types, conductive and nonconductive solutions, based on the property of the formulated solution to be filled. For the ampoule product filled with a conductive solution, the high-voltage leak detector is conventionally employed as a highly sensitive leak test method. In contrast, for an ampoule product containing a nonconductive solution, a distinct leak test method still needs to be developed for each product.
In the current study, a nonconductive solution is contained in glass ampoules and the ampoule headspace requires purging with nitrogen gas to prevent the oxidative degradation of the product component(s). In our case, the oxygen concentration in the ampoule head space is reduced to approximately 1% by replacement with nitrogen gas. To monitor the degree of the nitrogen replacement process during manufacturing, the laser-based head space analysis (HSA) method is widely employed to nondestructively measure the oxygen concentration in the ampoule head space. An increase of oxygen concentration in the ampoule headspace may be also a good indicator of potential ampoule defects relating to the presence of cracks. Thus, the HSA may be utilized to develop a novel leak test method for ampoule products filled with a nonconductive solution, such as an ethanol-based solution.
In this study, a new leak testing method utilizing HSA in combination with the bombing treatment was developed using ampoule products containing an ethanol-based solution. In addition, the proposed HSA-based leak test method was compared with a conventional dye ingress method for sensitivity in detecting ampoule defects during routine production. The purpose of this study is to define the effectiveness of the HSA-based leak test method for injectable products.
2. Materials and Methods
2.1. Materials
The HSA machine (WILCOMAT HSA-P, Lab tester, 090022) was obtained from Wilco (Wohlen, Switzerland). The dye ingress method was developed internally. The helium mass spectrometer (UL200) was obtained from Leybold Inficon (Koln, Germany). A 2 mL ampoule was obtained from Shiotani Glass Co., Ltd. (Osaka, Japan). Two types of defect ampoules were prepared as described in section 2.2.1. One defect was made with an inserted capillary, and the other is a crack defect. Capillary defects were used only for HSA bombing trials to show the effectiveness of the treatment. Other trials were conducted using crack-defect ampoules.
2.2. Methods
2.2.1. Methods for Preparation of Defect Ampoules:
Two types of defect ampoules were prepared using a 2 mL ampoule: one ampoule group includes those with an intentionally introduced crack and the other includes those containing a capillary tube of defined size inserted through the glass wall. To introduce the crack on the ampoule, the surface of an ampoule was cleaned, and then scratched with a diamond cutter at the point where the defect was to be introduced. The “scratched” ampoule was then heated using an oxyhydrogen burner, and subsequently a droplet of water was placed at the point using a micro syringe to cause heat shock leading to cracking. For the preparation of the ampoules with a capillary of defined size, at first small holes were introduced in the bottom of the empty ampoules before attaching the extensions which were used to connect the ampoules to the vacuum and purging mechanism. Capillaries with various lengths and diameters were then placed through the hole in the bottom of the ampoules and were immobilized and sealed with an epoxy resin. Two sets of capillaries were prepared. In one, each capillary was cut short to simulate the leak flow into the solution, and for the other each capillary was cut relatively long to simulate the direct leak flow into the headspace bypassing the solution. A helium mass spectrometer was then used to measure the actual leak rate for each set. The flow rate of helium gas was calculated at 1.1∗10|Lo–4 mbar∗l/s based on Poiseuille's Law under the condition where the diameter of a capillary is 1 μm, the length of capillary is 50 μm, and the differential pressure between inside and outside is 200 kPa.
The specifications of the two sets of capillaries to achieve these flow rates were as follows:
-
Capillary below solution = 12 mm length with internal diameter of 9 μm
-
Capillary into headspace = 38 mm length with internal diameter of 12 μm
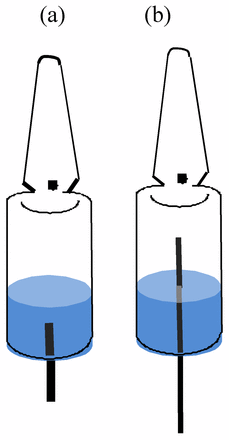
Both sets showed a measured leak rate of 1.1∗10∧–4 mbar∗l/s, which confirmed that a simulated defect of 1 μm is actually made. The ampoules were then filled with ethanol-based solution. The filled ampoules were sent to the vacuum and purging equipment. The ampoules were subjected to repeated processes of evacuation for complete purging with nitrogen. The ampoules were then flame-sealed and used for the ampoule leak testing (Figure 1).
Preparations of ampoule samples for the leak test. Two ampoule test samples, (a) and (b), were prepared: (a) 1 mL fill volume with simulated leak through solution (b) 1 mL fill volume with simulated leak into headspace.
2.2.2. Dye Ingress Method:
Conditions of the dye ingress method were basically set referencing the USP/EP method (6). However, the parameters of the study were not entirely in accordance with USP/EP method. Since methylene blue described in USP/EP was found to be incompatible with the ethanol-based solution, brilliant blue was selected for this study. In addition, the test chamber was pressurized instead of evacuated to match the same conditions as the HSA overpressure process. A comparison of the test method used for the current study versus the USP/EP method is found in Table I. The dye ingress method parameters were as follows: dye: 0.1% brilliant blue aqueous solution; test pressure: 200 kPa; pressure time: 30 min; time at atmospheric pressure: over 30 min; criteria: no blue dye observed in an ampoule.
Dye Ingress Methods
Only qualified operators engaged in visual inspection for this dye method. The operators were tested using the prepared samples with different levels of dye solution as outlined (Table II). In this qualification, 50 ampoules with dye solutions of several concentrations were randomly mixed with 200 ampoules free from dye solutions. Trained operators conducted visual inspection for the 250 ampoules, and their ability was demonstrated to detect 0.1 ppm level of dye solution.
Detectable Concentration of the Dye Solution by Sight
2.2.3. HSA Method:
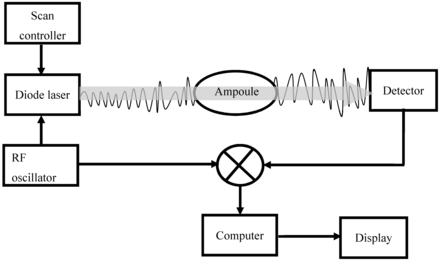
The principle of the HSA method is described in Figure 2. The HSA was used to measure oxygen concentration in the ampoule headspace. Before measurements, the HSA was calibrated using standard ampoules with known oxygen concentration (certified 1% oxygen gas). The following test parameters were set up on the HSA to conduct the trials: optical path through gas [1/100 mm]: 1201; number of scans: 400; minimum transmission [%]: 40; number of digits: 2; delay measuring [cycles]: 3.
Principle of HSA. A wavelength modulated low power laser beam (760 nm) is passed through the headspace of the ampoule, and the detector converts the incoming light into an electrical signal, and the strength of light is reduced at the point of the absorption. The absorption signal is then correlated to the number of oxygen molecules present in the ampoule headspace.
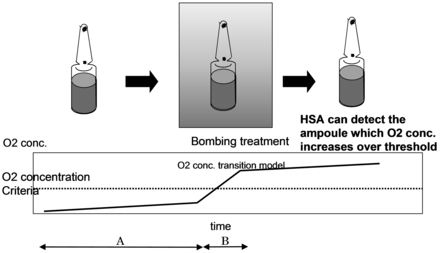
Using ampoules filled with 1.0 mL ethanol-based solution and low concentration levels of oxygen in the head space (usually around 1% in routine production operations), a defect ampoule can be identified by the HSA method based on the increased levels of oxygen in the headspace caused by diffusion of outside air through its crack over time. By pretreating with the bombing procedure (Figure 3) under the pressure condition (200 kPa for 30 min), oxygen ingress into the defect ampoule can be accelerated.
Concept of HSA as a leak tester. Oxygen concentration is low in the filled ampoule (approximately 1%) and the observed increase of oxygen in the headspace detected by the HSA is driven by a slow diffusion process of oxygen through the cracks (Process A). The pre-treatment known as the bombing process (subjecting the ampoules to overpressure) accelerates the process of the air ingress from the outside into the ampoule (Process B), which facilitates the increase of oxygen concentration in the headspace, allowing the HSA to detect the ampoule defects clearly.
2.2.4. Preliminary Evaluations of the HSA Method Used as A Potential Leak Testing Method:
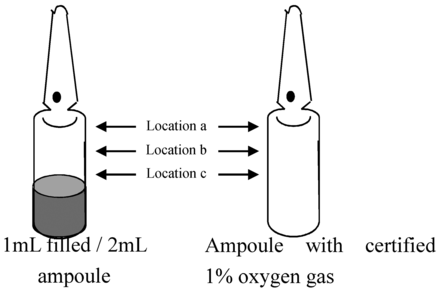
Three sets of tests were conducted on the HSA method for preliminary evaluations to confirm the robustness of the technology. They were to examine how liquid, ethanol-based solution on the surface of the ampoule affects measurement accuracy, to examine the uniformity of oxygen gas concentration in the ampoule head space (Figure 4), and to examine the effect of temperature on accuracy of measurement. The measurement was taken ten times for each experiment. In addition, HSA tests were performed on ampoules filled with various concentrations of oxygen to demonstrate method linearity, accuracy, and precision.
Locations of measurement to confirm the uniformity of oxygen concentration measurements in the ampoule head space.
2.2.5. Pretreatment of Samples with the Bombing Process:
To confirm the effect of the bombing process, this study was conducted using two types of ampoules with different capillaries. One set included capillaries cut short to simulate the leak flow into the solution, and the other set included capillaries cut relatively long to simulate the direct leak flow into the headspace, bypassing the solution. The test ampoules were measured by HSA to confirm initial oxygen concentration. The ampoules were then placed in the bombing chamber under 200 kPa pressure of compressed air. After the ampoules were removed from the chamber, the oxygen level was recorded by HSA at 5 min intervals up to 30 min in total.
2.2.6. Comparison Trial of HSA versus the Dye Ingress Test:
The study was conducted using ampoules manufactured in production, and artificial cracks were made on the ampoule surface. The initial oxygen concentrations in the test ampoules were measured by HSA. The ampoules were then placed in the bombing chamber under 200 kPa pressure of compressed air for 30 min. The ampoules were taken from the chamber and the oxygen level was measured again, then the dye ingress test was conducted. Finally the samples were visually checked after washing the outer surfaces of the ampoules.
3. Results and Discussion
3.1. Development of the HSA-Based Leak Test Method
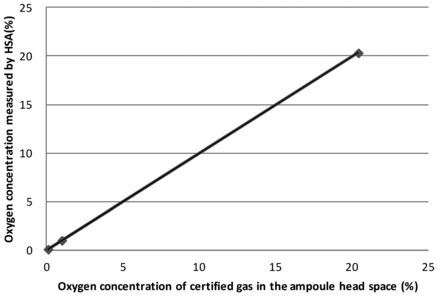
Basic trials using ampoules with certified 0.1%, 1.0%, and 20.4% oxygen gas were HSA tested to see the linearity, accuracy, and precision of the HSA measurement. Regarding the method's linearity for the full operation range, that is, between 0% and 20.4%, the coefficient of correlation was 0.999 (Figure 5). It was found that there was high linearity as the result of measurements. Also, the standard deviations of each measurement using the above certified ampoules were 0.06, 0.1, and 0.1, respectively, as the result of ten repeated measurements. The differences between the true value (certified oxygen gas concentration) and the average value were −0.02, 0, and −0.1, respectively (Table III). As a result of this basic trial, HSA has a potential to measure oxygen concentration with high linearity and accuracy.
Linearity of measurement by HSA using certified oxygen gas.
Oxygen Concentration by HSA Measurements Using Ampoules with Certified Oxygen Gas
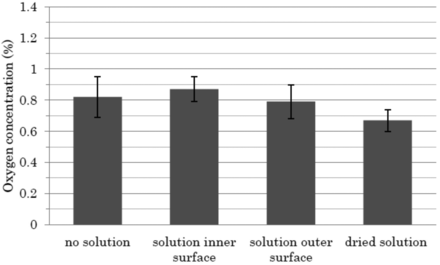
To examine the feasibility of the HSA-based leak test method, studies were conducted to see (1) the effects of the presence of ethanol-based solution on the outer or inner surfaces of an ampoule on the accuracy of HSA measurement, (2) uniformity of oxygen gas concentration in an ampoule head space, and (3) the effect of temperature on the accuracy of HSA measurement. The effects of the presence of solution droplets on the outer and inner surfaces of glass ampoules were examined using 1.0 mL filling volume/2 mL ampoule. The standard deviation of each measurement was approximatley 0.1, and the range of values was within approximately 0.1 from the average as well. The results indicated that the presence of the solution in the laser path does not affect the accuracy on the measurements of the oxygen concentration (Figure 6).
Impact on the overall accuracy of ethanol-based solution (the solution inside and outside the ampoule) in the measurement path. A single good 1 mL-filled/2 mL ampoule (no defects) was prepared and used to examine the effect of the presence of droplets of the solution on the outer and inner surfaces of the ampoule on the determination of oxygen concentration. At first, the oxygen concentration in the ampoule was measured by HSA in conditions where no droplets were present on the inner surfaces of the ampoule in the headspace region. The oxygen concentration was subsequently measured (n = 10). Following that, the same ampoule was inverted to simulate the condition where the solution is on the inner surface of the ampoule in the headspace region. The ampoule was immediately placed in the HSA before the solution adhered to ampoule head space wall reverted back to the body position and oxygen concentration in the head space was measured. For the test of the solution on the outer surface, the solution was applied at the position of headspace of the same test ampoule and the concentration of oxygen was measured ten times by HSA. Further, the solution on the outer surface of the same test ampoule was allowed to dry before taking the final set of measurements.
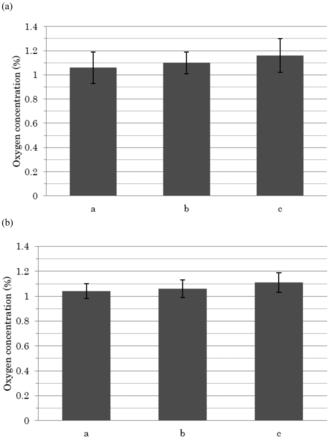
As a separate study, the uniformity of oxygen concentration measurements in the ampoule head space was evaluated. The standard deviation of each measurement was approximately 0.1, and the range of values was within approximately 0.1 from the average as well. This indicated the relatively high levels of uniformity of the oxygen concentration measurements within the ampoule headspaces (Figure 7a and 7b). In addition, ethanol vapors have no significant effect on oxygen measurement (Figure 7b).
Uniformity of oxygen gas concentrations in ampoule head spaces. An ampoule with only certified 1% oxygen gas (a) and a 1 mL ethanol-based solution-filled/2 mL ampoule with no defects (b) were used. The oxygen concentration was measured ten times at the three different positions (indicated with a, b, and c) in Figure 4.
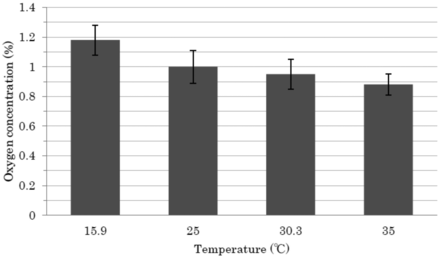
The oxygen concentration measurements were then conducted at varying temperature conditions (from 16 to 35 °C). The standard deviation of each measurement was approximately 0.1, and the range of values was within approximately 0.2 from the average, indicating that the surrounding temperature could affect the accuracy of oxygen measurement in the headspace but the degree could be very limited (Figure 8), given that generally the temperature range in a production area is controlled at approximately 22 ± 3 °C.
Effect of temperature on accuracy of measurement. A single good 1 mL-filled/2 mL ampoule (no defects) was used. Each ampoule was kept for 20 min at each different temperature (16 °C ∼ 35 °C) to reach equilibrium before measurement. The measurement was repeated ten times. The average values of oxygen concentration with the standard deviation were calculated and plotted against each temperature in the graph.
In summary, these feasibility study data support the robustness of the HSA method for leak detection.
3.2. Effect of Bombing Treatment
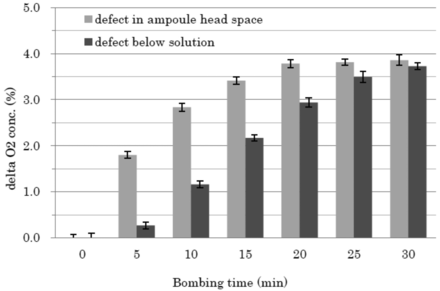
The bombing treatment is the process used to accelerate oxygen ingress into defective ampoules by the overpressure generated. To confirm the effect of the bombing treatment, the test ampoules each with a capillary of known diameter to simulate a defect of a 1 μm orifice pinhole were prepared and the initial oxygen concentration was measured by HSA. The ampoules were then placed in the bombing chamber under 200 kPa pressure of compressed air. Then, the ampoules were taken from the chamber and oxygen level was measured again every 5 min up to 30 min in total. The results indicated that oxygen concentrations were rapidly elevated with time during the bombing treatments and ultimately reached a plateau (Figure 9). The results show the usefulness of the bombing treatment for accelerating the oxygen ingress into the ampoules.
Effect of the bombing process on the increase of oxygen concentration in ampoules with capillaries. The oxygen concentrations of two test ampoules, 1 mL-filled/2 mL ampoule with capillary, were measured to confirm the initial oxygen concentrations. The ampoules were then placed in the bombing chamber under 200 kPa pressure of compressed air up to 30 min. The ampoules were taken from the chamber and the oxygen level was repeatedly measured every 5 min for 30 min in total. Each measurement was taken ten times. Data are processed to obtain the difference of oxygen concentrations before and after the bombing process, that is, delta oxygen concentration, and plotted against the bombing time. (a) Cracks in the ampoule head space. (b) Cracks in the ampoule body.
According to the principles of the HSA method, as the temperature is raised, the apparent level of oxygen concentration will increase due to the excited motions of an oxygen molecule. On the other hand, the apparent oxygen concentration decreases as the pressure is raised, because the oxygen's molecular movement is inhibited. However, from results of 3.1 and this bombing study (3.2), these factors showed little effects on the accuracy of oxygen measurement in our studies under the pressure (up to 200 kPa) and temperature (16–35 °C) conditions employed. It is also noted that the rate of oxygen concentration is affected by the location of a defect, that is, above and below the solution level. So, as described hereinafter, a comparison study between HSA and dye ingress was conducted using both crack defects, that is, above and below the solution level.
3.3. Comparison between HSA as A Leak Test and A Dye Ingress Method
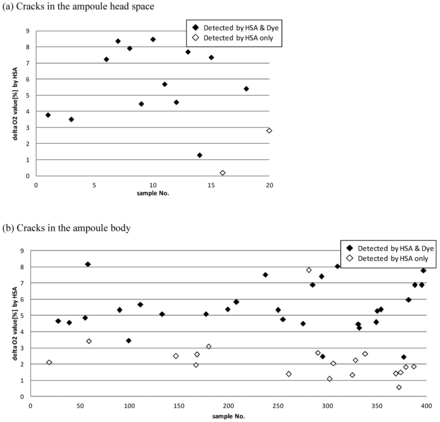
To compare leak detection sensitivity between a dye ingress method and the HSA method, studies were conducted using ampoules with a crack in the ampoule head space region and in the ampoule body, that is, below the solution level. It is speculated that the process of oxygen diffusion through a crack proceeds slowly when the crack is present below the solution level of an ampoule. Therefore, it was particularly necessary to evaluate the sensitivity of HSA under this condition. A larger population of ampoules was therefore subjected to the experiment to compare the detection sensitivity between the dye ingress method and the HSA method. The delta oxygen concentrations, defined by the difference of the oxygen concentration before versus after the bombing procedure, are plotted for those samples detected only by the HSA method, and for those test samples detected as leaking by both the HSA and the dye ingress method when the crack is located in the ampoule head space (Figure 10a). It should be noted that there were two samples that could be detected by the HSA but not by the dye ingress method. Similar results are shown in Figure 10b for samples simulating cracks in the ampoule body. It should be noted that the dye ingress method could detect only 29 ampoules compared to 48 ampoules detected by HSA. It is noteworthy that assuming the delta oxygen concentration increases with increasing crack size, lower values are indicative of higher sensitivity in detecting the change of oxygen concentration. Thus, it appears that the HSA method offers higher sensitivity than the dye ingress method for ampoules with cracks located above the solution level and on the ampoule body.
Comparison of sensing capability of the HSA method and the Dye ingress method. Twenty ampoules (1 mL ethanol-based solution-filled/2 mL ampoule) with cracks in the head position of an ampoule are prepared (a). In addition, 400 ampoules (1 mL ethanol-based solution-filled/2 mL ampoule) with cracks in the body position of ampoule were prepared (b). The initial oxygen concentrations in the ampoule head space were around 1% before making the cracks. For each ampoule, the HSA test in combination with the bombing process (bombing pressure: 200 kPa, bombing time: 30 min) was initially conducted followed by the dye ingress test (dye: 0.1% brilliant blue aqueous solution, test pressure: 200 kPa, pressure time: 30 min). Sample populations are shown in (a) and (b) for cracks in the head and cracks in the body, respectively, on the representative ampoule defects which show the HSA result such as the increased level of the oxygen concentration (more than 1.0%) or on the absolute oxygen concentration after the bombing treatment (more than 2.0%). Data means the difference of oxygen concentrations before and after the bombing process, that is, delta oxygen concentration.
Based on the results from the above feasibility studies it was found that the HSA method offers greater leak detection sensitivity compared to the dye ingress method for ampoules containing ethanol-based solution and with nitrogen gas in the head space, irrespective of the location of the cracks.
4. Conclusion
The data presented indicates the potential applications of the HSA method as a leak test for glass ampoule products filled with ethanol-based solutions. This technique can theoretically be applied for lyophilized products in vials when their head spaces are replaced with nitrogen gas as well. The potential advantages of the HSA method over the blue dye method include the relatively high sensitivity and nondestructiveness of the HSA method. However, there are limitations for the HSA method in that it can be applied only for a product with purged nitrogen gas in the head space. In addition, it is necessary not only to optimize the bombing process for the product of interest before application in production, but also to develop the overall process in consideration of the traffic lines for personnel and materials. However, more interestingly, the HSA method has the potential of being implemented as a high-sensitivity, 100% automatic inspection system for ampoule products.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2012