Abstract
The major compendia require sterile injectable and ophthalmic drugs, to be prepared in a manner that is designed to exclude particulate matter. This requirement is satisfied by testing for subvisual particles in the laboratory and 100% inspection of all containers for the presence of visible particles. Inspection for visible particles is performed in the operations area using one of three methods. Manual inspection is based on human visual acuity, the ability of the inspector to discern between conforming and nonconforming containers, and the ability to remove nonconforming units. Semi-automated inspection is a variation of manual inspection, in which a roller conveyor handles and presents the containers to the human inspector. Fully automated inspection systems perform handling, inspection, and rejection of defective containers. All inspection methods must meet the compendial requirement for sterile drug product to be “essentially free” of visible particulates.
Given the random occurrence of particles within the batch, visual detection of a particle in an individual container is probabilistic. The probability of detection for a specific particle is affected by many variables that include product attributes, container size and shape, particle composition and size, and inspection capability. The challenge set is a useful tool to assess the particle detection in a product, and it may also be used to evaluate detection of container/closure defects. While the importance of a well-designed challenge set is not always recognized or understood, it serves as the cornerstone for qualification and/or validation of all inspection methods. This article is intended to provide useful information for the design, composition, and use of container challenge sets for particulate inspection studies.
LAY ABSTRACT: Regulations require drug products intended for injection or ophthalmic use to be sterile and free of particles that could harm the patient. This requirement is meet by 100% inspection of every drug container in the lot and the removal of any defective unit before it is released for patient use. Great progress has been made through the creation of a harmonized method for the detection of small particles in drug product and universal recognition of container defects.
Differing opinions concerning the conduct of large particle inspection have hindered the creation of a harmonized method. The absence of a standard method has created confusion that must be resolved for acceptance of drug products in the global marketplace. While the importance of a well-designed test set for qualification of these methods is overlooked or misunderstood, it can serve as the cornerstone for qualification of all inspection methods. This article is intended to provide valuable information for test sets used qualify inspection systems. The proper design and use of these test sets will provide clarity for inspection qualification, which can be applied to the inspection of commercial product.
- Challenge sets
- Container/closure defects
- Inspection validation
- Kits
- NIST spheres
- Particle inspection
- Particulate matter
Introduction
Inspection of product in the operations area is primarily focused on container/closure defects and particulate matter in product. Both type defects present potential harm to the patient; however, the circumstances surrounding their detection and rejection are very different. Most container/closure defects are easier to detect than particulate matter in product and guidance provided by the Parenteral Drug Association (PDA) and the U.S. Food and Drug Administration (FDA) has enabled companies to create clearly defined acceptance criteria and disposition for each type of container/closure defect (5–7).
The major compendia have harmonized the testing methodology and acceptance criteria for subvisible particles (1–4); however, the absence of a harmonized guidance for “visible particles” has led to confusion in the global industry. The wording may vary, but the major compendia share the vague expectation for injectable and ophthalmic products to be “essentially free” of visible particulate matter. This expectation is based on human visual acuity, which is subjective and can be affected by many variables. In the absence of a harmonized guidance, this review article is based on published studies and experience gained in the design and use of challenge sets for particulate matter studies.
Manual Inspection:
The pioneering work of Knapp and Kushner in 1980 introduced an inspection methodology that minimized manual inspection variability and provided a statistical means to measure inspection performance (8). Controlled manual inspection continues to be a practical means to detect visible particulate matter. The USP, the Code of Federal Regulations, and the FDA provide minimal information regarding the conduct of particulate inspection. Section 2.9.20 in the European Pharmacopeia (EP) provides the most definitive guidance for manual inspection methodology, which is consistent with many documented studies. In the absence of a harmonized guidance, the EP provides a sound basis for qualification of manual inspection. Although human visual inspection may be affected by many variables, published data indicates that most of these variables can be controlled to provide reproducible data (8–19).
Semi-Automated Inspection:
Many companies use semi-automated systems, installed in-line with filling or packaging operations to assist with container/closure inspection. Filling and packaging lines typically run at speeds ≥100 units/min and inspection must be qualified using representative defects and the same conditions intended for the inspection of commercial product. The inability to qualify in-line particulate inspection at excessive line speeds, is the reason why of the injectable drug manufacturers that participated in the latest PDA survey, only 24% perform particulate inspection in-line (20). In-line inspection for visible particles under these conditions compares unfavorably to manual inspection performed in an inspection booth, having a median inspection time of 5–6 seconds against each color background (20). Semi-automated inspection systems may be qualified for particulate inspection, when installed off-line, with additional inspection time.
Automated Inspection:
There is increased use of fully automated inspection systems that perform both container/closure and particulate inspection in a single pass. Data from numerous studies has proven that automated inspection systems are more sensitive and consistent in detecting rejects than manual inspection. Automated inspection settings are dependent on the physical properties of the product, container, fill level, and the particle type and size. It is essential that the challenge set has the same attributes as the commercial product and that it contains a sufficient number of defective units to provide an accurate assessment of process capability.
The Role of Challenge Sets for Inspection Qualification/Validation
Challenge sets have many uses. They can be used to train inspectors, qualify and requalify inspectors, and establish a manual performance baseline. The manual baseline can be used to validate alternate inspection methods, evaluate changes to the inspection process, or perform preproduction functionality testing of an automated inspection system. In our experience, the size and scope of a challenge set may expand with experience, and useful information that is gained can lead to process improvements. Challenge sets provide a measurable link between the probability of detection (POD) for various particle types and sizes. All challenge sets contain a subset of defect-free containers and a subset of containers having one known defect. The challenge set, in combination with predetermined acceptance criteria and a sound inspection strategy, enables qualification/validation of inspection methods designed to differentiate between defect-free versus defective containers.
The principles for the design and use of a challenge set apply to manual qualification and validation of automated systems for particle detection or container/closure defect detection. During inspection of commercial product, container/closure defect and particulate inspection may be performed in a one- or two-step process. Inspection for container/closure defects involves slow rotation of the container to examine all surfaces, while particulate detection requires a greater rotation speed to put particles in motion. Maintaining separate data and calculations for each type of defect is essential to gain an understanding of both aspects of inspection. Cherris supported this position at the 2011 PDA Visual Inspection Forum (9).
Considerations in the Design and Use of a Challenge Set
Shelf Life:
It takes a considerable amount of time and resources to manufacture a quality challenge set. Borchert and other investigators quickly realized the value of having a stable challenge set (10, 11, 13, 14, 22, 27). Challenge sets intended for inspector qualification/requalification and establishing a manual baseline must be stable and have reproducible results for long-term use. Aqueous challenge sets that are filled aseptically with a stable solution and compatible container/closure typically have a useful shelf life of 3 to 5 years.
Challenge Sets for Manual Inspector Training and Qualification:
A challenge set used to qualify/requalify manual inspectors or to establish an in-house process capability must be stable and capable of withstanding repeated handling over time. Many investigators have recognized the importance of using a well-designed challenge set for inspector qualification and to establish a statistically sound manual baseline for validation of automated systems (8, 9, 11, 13, 14, 21–23). Challenge sets intended for manual inspection studies must contain particles representative of those found in commercial product (e.g., glass, rubber, steel, etc.) that are within the range of visual acuity. The particles may be sourced from actual manufacturing rejects or may be simulated particles manufactured in a laboratory. A stable challenge set can also be used to evaluate process drift or measure the effect(s) of a change to the inspection process (21).
Product Characteristics:
“Know thy product” seems overly simple, but product-specific attributes such as viscosity, specific gravity, surface tension, tendency to form air bubbles, head space, terminally sterilized products containing schlieren lines (stratified layers of different density), and other factors can directly affect inspection methodology and the composition of the challenge set. Drug product attributes can significantly affect inspection methodology, and they must be understood before designing a challenge set (23–30). Studies by Rathore, Sing, Shnek, Gidth, and Deng provide a great deal of detailed information concerning the characteristics of biopharmaceutical products and the idiosyncrasies of inspecting these products (26–30). Rathore et al. discussed in detail how the viscosity, density, and surface tension of biopharmaceutical formulation could affect particle detection with automated inspection (26). Singh et al. stressed the importance of a stable formulation and how protein aggregates ≤50 μm may lead to visible particle detection (27).
A subvisual particle analysis of a biopharmaceutical or complex bulk formulations can provide valuable information regarding the enumeration and characterization of particles inherent to the formulation (33). This is especially useful for products that have opalescence, turbidity, suspensions, and smaller particles that may join to form larger (aggregated) particles. Additional testing for subvisual particles in the product, after it is filled and sealed, may provide insight into external particle contribution and potential interaction between the formulation and container/closure. Having this knowledge before beginning visual inspection studies can save a great deal of time and failed studies. The scope of this article does not permit going into further detail regarding biopharmaceuticals or other difficult-to-inspect products; however, the reference article by Singh et al. provides detailed information regarding the best practices for formulation and manufacturing of biotech drugs (26). Expertise with orthogonal particle investigational methods has grown at laboratories with new instrumentation, and future studies should provide more detailed information on the inspection of biopharmaceutical products.
Container Configuration:
Container configuration and the material of construction can affect the ability to detect particles. Amber glass and opaque plastic containers require increased light intensity and/or increased camera gain to detect particles. When increased light intensity or increased inspection duration is used to inspect photosensitive products, the same inspection method intended for commercial inspection should be used to inspect stability samples to ensure that product stability is not adversely affected by enhanced inspection lighting. By constructing a small challenge set with each of the container sizes and fill volumes, with specifically designated particle sizes and types, the differences in POD and other inspection variables can be better compared with statistical confidence. Syringes present challenges due to their small-bore size, which provides less room to form a vortex during spinning.
Studies by Shnek et al. experienced problems with standard spheres sticking to the interior surfaces of prefilled syringes filled with biologic products (28). These studies indicate that physical properties of the particle can affect detection in syringes. The studies also included the seeding of syringes with one, versus multiple, spheres (28). Gidh et al. approached the problem of inspecting viscous liquids in prefilled syringes by suspending particles in varied formulations using NaCl, polyethylene glycol (PEG), water for injection (WFI), and 5% polysorbate to increase particle suspension and detection (29). Deng et al. took a similar approach by using a placebo solution that mimicked the properties of the drug product. Their findings concluded that particle detection is more difficult in syringes filled with viscous solutions, which tend to generate air bubbles during spinning (30). Unique (undetectable) methods have been introduced to seal the needle path to prevent particle loss.
How Many Containers Belong in the Challenge Set?:
Knapp recommended the use of a challenge set consisting of 250 containers in his initial validation procedure (8). The absolute number of containers in the challenge set is not as critical as having a sufficient number of containers that represent the range of particle types and sizes required to provide a statistical assessment of process capability. The design of experiment (DOE) and end-uses of the challenge set should determine the number of required containers in the set.
Particle Size and Type:
What particle sizes and types should be seeded into a challenge set container? There is no universally accepted requirement for specific particle sizes to be included in inspection studies. When considering the overall range of particle sizes, 50 μm is accepted as the threshold for a human inspector having 20/20 visual acuity. This value is often misunderstood, as a 50 μm particle may only be detected 0–3% of the time with multiple inspections. The inclusion of a 50 μm particle adds little value for routine qualification, but it may provide confidence in studies designed to evaluate the lower range of visual detection.
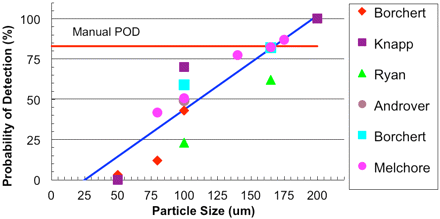
In the search for a quantitative approach to validation, much attention has been given to particle size as the criterion that contributes to particle detection. Other particle attributes including shape, composition, reflectivity, color, contrast, density, and particle behavior during spinning and inspection must also be considered. Glass shards will tend to twinkle due to the edges of the particle, while this is not seen with a NIST (National Institute of Standards and Technology) glass sphere. Container type, size, shape, and fill volume affect the apparent size of a particle and must be considered when selecting particle sizes for testing. The studies referenced in this article indicate that one particle size range for manual inspection may not be suitable for all product/container combinations. Particle sizes and types to be seeded into challenge set containers are determined by the end-use of the set, and they should be specified in the DOE. The particle sizes used in Figure 1 serve as a practical guideline for studies conducted with small volume clear glass containers (12). For certain containers, this may not be evident. A limited, small inspection study, using a few challenge containers with small sizes, may provide useful information for the overall particle size range in the DOE.
Probability of detecting particle sizes in small volume clear glass containers (12).
Dunham conducted manual inspection studies utilizing a light intensity of >500 foot-candles (>5382 lux), combined with a 15 s inspection duration, to determine the POD for NIST-traceable spheres in 20 mL glass vials, 250 mL glass bottles, 50 mL flexible containers (infusion bags), and 250 mL flexible containers (31). Detection of a 163 μm particle was comparable to earlier studies reported by Shabushnig and Melchore, using light intensities ranging from 225 to 375 foot-candles (12). In contrast, Budd reported a 93% POD for detecting a comparable sized particle when using a light intensity of 5500 lux (36). These studies serve as a useful guideline for conducting SVP inspection, with the understanding that there will be site-to-site variation dependent on operational differences. Dunham's data also indicates there is little to be gained by increasing particle size once a given particle size is detected with approximately 90% reproducibility in 20 mL vials.
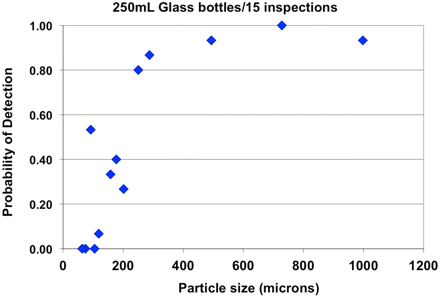
The data in Figure 2 taken from Dunham's studies conducted with a 250 mL bottle indicate that the same sized particle detected in the 20 mL vial had a significantly lower POD when seeded in a 250 mL bottle. The larger inspection window and increased depth of field of the 250 mL bottle affects the apparent particle size to the manual inspector, which is evidenced by a decreased POD. Automated inspection machines are designed to inspect multiple sized containers, and container size has less impact on automated systems.
Probability of detecting particles in a 250 mL bottle (31).
POD Calculation:
The challenge set must be inspected multiple times to develop a statistical POD for each container in the set. Knapp's original validation procedure recommended at least five (5) inspections of each container by multiple inspectors for a total of 70 inspections to provide data with a 95% confidence limit. Due to the probabilistic nature of particle detection, statistical confidence in the POD increases with the number of inspections, and investigators referenced in this article have utilized >10 inspections to calculate the POD. At some point practicality limits the number of manual inspections that can be performed, and a risk assessment, conducted by a multi-disciplinary team, provides a sound basis to determine the number of inspections to be performed.
Statistical Subsets:
The Knapp validation procedure divides the challenge set into three subsets based upon the POD, which is calculated by a minimum of 20 inspections (9). The containers are placed into one of three subgroups based on their POD:
The Accept subset consists of 170 containers that have a rejection probability ranging from 0 < 0.3. It represents ≈68% of the challenge set.
The Grey Zone subset consists of 40 containers that have a rejection probability ranging from 0.3 < 0.7. It represents ≈16% of the challenge set. Grey zone containers are not true rejects, but are containers that are sensitive to subtle changes in the inspection process. They provide security that the reject containers are detected.
The Reject subset consists of 40 containers that have a rejection probability ranging from 0.7 ≤ 1.0. It represents ≈16% of the challenge set.
With effective particulate control procedures in place, inspectors routinely experience rejection rates ranging from 1 to 2% during inspection of commercial drug lots. Great care must be taken when designing challenge sets to avoid positive reinforcement (sensitization) of human inspectors, as challenge sets typically have a greater percentage of defective containers than with commercial lots. Knapp reported positive reinforcement when the Reject subset exceeded 25% of the challenge set (8). Many studies reported in this article have taken a more conservative approach and kept the reject subset between 20–25% of the total containers in the challenge set, for studies involving human visual inspectors (11, 12, 14, 15, 19, 20, 22). Contrary to urban myth, automated inspection systems are not subject to sensitization. The proportion of rejects in the challenge set or the order in which the containers are inspected does not influence the results with an automated inspection system because these systems are not subjective.
Preproduction Functionality Test Automated Systems:
Typically, companies have a procedure to verify and document automated system settings prior to inspection of commercial product. Many companies also perform a preproduction inspection of a challenge set that contains sufficient acceptable and reject containers to ensure that each station of the automated system is functional. This type of challenge set may consist of a portion or the entire validation challenge set, and it provides added security of machine function (20).
Seeding Containers:
Independent studies performed by Borchert, Shnek, and Gidh compared the effect on POD when containers were seeded with one versus multiple NIST spheres (10, 27, 28). Their independent findings confirm that seeding containers with more than one particle will result in an artificially increased POD. This is due to a multiplying effect of two smaller particles in proximity being detected as one large particle, and the identity of the particle that was detected will be unknown. The practice of adding more than one sphere was started to compensate for spheres that went undetected due to particles adhering to the interior syringe surfaces. In such cases, other corrective measures should be taken to prevent particles from adhering to interior surfaces. Other investigators referenced in this article stress the importance of seeding each container with only one particle in order to determine if a specific particle type and size was detected (11, 14, 26, 36).
Containers may be seeded with intrinsic particles found in the manufacturing process, extrinsic particles that enter from the environment, or simulated particles prepared in a laboratory that react in the same manner as natural particles during spinning and manual inspection. A variety of suitable materials have been identified for container seeding (20–25):
Fibers: fibers in challenge sets should include fibers from materials that are found in the production process. They can be either natural or synthetic. Commonly found materials are cotton, rayon, nylon, polyester, filters (polypropylene, Teflon® fluoropolymer resin, Teflon® fluorinated ethylene propylene), wipers, garments, and others.
Glass shards or glass lamella of the specific product/configuration.
Rubber fragments from stoppers; diaphragm pumps or “O” rings.
Stainless steel shards from manufacturing or filling equipment.
Challenge Sets Made from NIST-Traceable Spheres versus Real Particles
Challenge sets may also be prepared by seeding containers with NIST traceable spheres in lieu of actual particles. Standard spheres are manufactured from glass, polystyrene latex, stainless steel, or various resins of uniform shape and size. Standard spheres provide a quantitative measurement of size due to their uniform shape and consistency and have limited variability compared to real particles. Unlike real particles, they do not break down with repeated spinning and are suitable for extensive use during factory acceptance testing, site acceptance testing, and supportive engineering studies. Various sized spheres enable the plotting of a calibration curve, which provides a direct correlation between POD and specific particle sizes (15, 34–37). The calibration curve is useful for comparing inspection methods, inspector groups, or evaluating changes to the inspection process. In addition, spheres are suitable for long-term usage because they do not breakdown with repeated spinning like some natural particles.
McCormick and Bariexca evaluated particle detection with vials and syringes seeded with Type I USP borosilicate glass shards or NIST-traceable spheres of varied sizes (31). The data in Figure 3 indicates that glass shards were more readily detected than comparably sized standard spheres when inspected on the Eisai Automatic Inspection Machine (AIM). The dynamics of putting a sphere into motion are very different than with natural or simulated particles that have edges. Also, standard spheres may sink quickly after spinning, which provides less detection time.
Detection of glass shards versus glass spheres (32).
Although standard spheres have many desirable characteristics, they have minimal utility for training and testing manual inspectors. The physical appearance of standard spheres does not prepare manual inspectors for detection of real particulate matter found in commercial product. Also, the dynamics of putting a sphere into motion and its behavior after spinning are very different than with irregularly shaped particles. These differences affect both manual and automated inspection. Optimized settings for automated inspection of commercial product can only be achieved with real particulate matter found in product (17).
One of these studies revealed a common problem with syringe inspection. Two of 10 syringes, all seeded with a standard sphere, went undetected in 50 inspections, while the other eight syringes in the same group were detected in all 50 inspections. It was determined that the sphere was hidden in the syringe tip cap on these two syringes (a new technique is now used to plug the needle path). It is important to inspect each syringe after particle seeding to ensure that the sphere or particle remains visible during inspection. Once this problem was realized, creative methods have been used to prevent particles from going undetected.
Matrix Grouping for Qualification/Validation of Multiple Products
The creation of an individual challenge set to qualify or validate the inspection of every drug product is time consuming, costly, and may be unnecessary. The matrix approach places product/container combinations into groups that have comparable physical properties. The matrix approach can reduce the number of challenge sets required for validation of multiple products. One representative drug product is used to validate each drug product/container group; however, this approach is only possible when the representative drug product has the same physical properties as the other drug products in this class. The key requirements for products placed in the same group are that a suspended particle must react in the same manner during spinning, braking, and inspection. An example of a product grouping would consist of three aqueous products that have the same physical appearance, viscosity, container, fill volume, and inspection characteristics. If all attributes are comparable, one drug can be used to validate the inspection of the drug product group in the matrix.
Before considering the details of any proposed challenge set, the purpose of the challenge set must be described and the specifications for the containers must be determined. This is best accomplished by a risk analysis of the key issues and concerns (risks) for each segment of the inspection process. The outcome of the risk analysis should lead to design qualification or a user requirement specification to define the uses of the challenge set, as well as the particle sizes and types to be used. When all these considerations have been identified, defined, and evaluated, the particle types, sizes, and characteristics can be specified for inclusion into the challenge set.
Preparation of Specific Challenge Sets
Certified Standard Challenge Set:
This type of challenge set is designed for frequent handling over long time periods and is expected to have a stable shelf life from 3 to 5 years. It provides the greatest amount of information regarding the inspection process because each particle size and type is known, which enables a more exact comparison of particle size and POD. Certified standards are suitable for qualification/requalification of manual inspectors, establishing a manual performance baseline or validation of automated systems for inspection of aqueous products.
A compatible container/closure is selected and all components are washed and sterilized prior to use. Sterilized components are meticulously rinsed with WFI under a high-efficiency particulate air (HEPA) filter hood, using aseptic technique. The rinsed containers are filled with WFI (with or without preservative), a proprietary formulation having bacteriostatic properties, or a stable bulk drug product. The liquid is filtered prior to filling the containers to minimize the chance of any subvisible particle >25 μm from entering the container, as subvisible particles may attach to each other or be in close proximity at the time of inspection and interfere with the intended contents of the challenge container. If the container is to be particle-free it is immediately sealed after filling. Filtering is a desirable step in preparing challenge sets filled with biopharmaceutical products. However, filtering some large-molecule formulations can remove smaller particles but may cause aggregation or result in particles that may interact to form larger particles. Another alternative for making challenge sets with biopharmaceuticals is to use a placebo solution. The key to success is to select a placebo that looks like and behaves in the same manner during spinning and inspection as the actual product.
Reject containers are seeded with one particle or sphere of a known composition and size before being sealed under aseptic conditions. After sealing, the presence of the particle in the container is verified and the seeded containers are randomly distributed throughout the challenge set. All containers receive a permanent unique number, with the container contents recorded in an inventory list. The advantage of using this type challenge set is that the composition and size of each particle in the containers is known, verified, and documented.
Manufacturing a precision challenge set is time-consuming and tedious, and these precautions are required to create a challenge set suitable for inspector qualification over a number of years. Once received, proper storage of the challenge set is required for maximum shelf life.
Characterized Challenge Set:
Containers may be filled with diluent or bulk product in the laboratory, or with the contents of containers taken from the filling line. As with the certified standard set, only containers free of visible particles are selected for further work. Because the solution was not filtered prior to filling, the subvisible enumeration and sizes of the particles are unknown. Freedom of visible particles must be verified by multiple inspections to determine the POD for each container. Only containers free of visible particles can be aseptically seeded with a particle of known composition and size in the laboratory. As with the certified standard set, knowing the particle type and size enables a direct correlation between particle size and POD, but the effect of subvisible particles on POD is unknown. The characterized challenge set is useful for nonbiologics or formulations known to have few subvisible particles.
Challenge Sets Using Production Line Containers (Knapp Boot Strap Method):
This challenge set utilizes containers filled with drug product taken directly from the filling line/inspection area to serve as the basis for a challenge set. The incorporation of containers taken directly from the inspection booth or filling line can not be directly placed into a challenge set unless they are inspected multiple times to provide a POD. The use of containers removed from the production line becomes less of an issue as the number of inspections is increased, as there is greater confidence in the POD. This method has had mixed success with some products due to small particles in the formulation, which have the potential to come into contact with each other or be in close proximity during inspection. The POD is based on multiple inspections, without knowing the particle composition or size. Qualification of individual inspectors compared to the inspector pool is possible with this type of challenge set, as the POD of the individual inspector is compared to the POD of the inspector pool. The manual inspection baseline may also be used as a basis to validate an automated inspection system. The disadvantages of this approach include not being able to correlate POD directly to particle size. In addition, the composition of some particles may be unknown and the potential effect of subvisible particles on the POD will be unknown.
A great deal of time can be saved if an experienced technician or inspector examines the containers taken from the production line and examines each container carefully before creating a challenge set to be inspected multiple times by the inspector pool. Using increased light intensity, longer inspection duration, and a useful device such as the Eisai APK, which spins, brakes, and enables inspection of the container with enhanced lighting. These added steps can facilitate improved selection of containers for the challenge set.
Challenge Sets Containing Toxic or Potent Compounds:
Custom-filling each visual inspection standard in the laboratory with a drug product can only be performed when the product is not harmful to humans, as the steps involved in filling and seeding several containers results in prolonged exposure for the microscopist. Drug exposure can be minimized by using drug-filled containers taken from the production line as described under uncharacterized challenge sets or by the use of a placebo solution that mimics the physical properties of the drug product. The use of a placebo solution offers a safe alternative for laboratory personnel preparing containers with toxic or potent drugs. It is critical that the placebo formulation has the same physical properties of the finished product and that particles suspended in the placebo solution must react in the same manner as the actual drug product, from the time the placebo solution is manufactured until the inspection study is completed. As with the Knapp Boot Strap Method, containers taken directly from the filling line and inspection booth must be carefully examined under optimized conditions before they are placed into a challenge set.
General Comments Regarding Challenge Sets:
Each time this challenge set is used, the current test data should be compared with historical data in order to determine if the current classification of a container is consistent with historical data. During this review, any container that has a significant change in classification should be closely examined, characterized, or removed from the set. As noted by Leversee and Shabushnig, challenge sets should be inspected periodically for microbial growth, change in product appearance, or damage to the container/closure (20). A written standard operating procedure, specifying a required container examination review period, with documented findings, provides a means to ensure that the challenge set has consistent performance.
When constructing a challenge set, it is prudent to have additional containers prepared for each subset to replace broken or unusable containers through repeated use. This is particularly important for rare or specific in-house manufacturing particles that occur less frequently. Challenge sets must be stored in a secure and controlled environment.
Containers having any unidentified particles should be sent to a qualified laboratory for analysis. Understanding the composition of each particle in the challenge set provides a better understanding of the inspection process and can be used to determine the root cause of particle generation.
Summary
Inspection of filled drug product containers serves as a monitor of particulate contribution from upstream manufacturing processes. Inspection does not add quality to the product, but it does provide a checkpoint where nonconforming containers can be detected and rejected from the lot. As the industry strives toward manufacturing excellence, technological improvements such as barrier technology will reduce the particulate load in product. The latest PDA survey indicates that many manufacturing operations have achieved particulate rejection rates between 1 to 2% and that exceptional operations having particulate reject rates ≤1% (20).
Although this article is concerned with challenge sets, issues with the absence of a harmonized methodology for visual inspection need to be evaluated. A harmonized methodology for subvisual particulates exists, but there is great disparity concerning methodology and acceptance criteria for “visible particles.” The major compendia is based on the detection of large particles using visual inspection. However, none of the compendia have a requirement for inspector visual acuity. Data published by Knapp indicates that the difference in particle size detection between 20/20 and 20/30 visual acuity is the ability to see a 58.2 μm particle versus a 87.3 μm particle (35). Requirement for 20/20 visual acuity is essential for visual inspection (natural or corrected). PDA surveys verify that most companies have adapted this requirement (20). The authors recommend that 20/20 visual acuity become a universal requirement. We base this recommendation on numerous studies, industry practice, and optical data concerning contrast thresholds of the human eye that were published as far back by Blackwell in 1946 (38).
Differing opinions concerning light source, light intensity, and methodology are likely to continue. In addition, the variability of products in the market requires different inspection techniques, and this has hindered the creation of a harmonized inspection method. The guidance provided by the EP has the essential elements of visual inspection, and the light intensity specified in the EP has been used in most of the referenced studies. The only missing elements in this guidance are a specified time for inspection and technique. If Knapp's recommendation for a 5 s inspection in front of each color background is added, the EP guidance could serve as a reasonable interim document until there is a harmonized method, and this might reduce differences in product acceptance in the global marketplace.
Regulatory agencies and industry want to see a qualitative number replace the vague acceptance criteria of “essentially free” from visible inspection. However, the studies referenced in this article indicate that particle detection is affected by many variables. In the past, visible inspection and subvisible particle compliance have been considered separately, but recent studies concerning particles in biotech and other products indicate that both regions (all particle sizes) must be considered collectively. The present acceptance criteria can be further defined for each product by establishing in-house performance criteria through the use of challenge sets and can bring different products and methods closer to harmonization. The authors hope that this article has clarified questions concerning challenge sets and that we have emphasized the important role that they play in the inspection process.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2012