Abstract
This report deals with the construction and management of the reverse osmosis (RO) water system for final rinsing of surgical instruments in the washer-disinfector. Numerous operational challenges were encountered in our RO water system and these were analyzed utilizing the Ishikawa Fishbone diagram. The aim was to find potential problems and promote preventive system management for RO water. It was found that the measures that existed were inappropriate for preventing contamination in the heat-labile RO water system. The storage tank was found to be significantly contaminated and had to be replaced with a new one equipped with a sampling port and water drainage system. Additional filters and an UV treatment lamp were installed. The whole system disinfection started 1.5 years later using a peracetic acid–based compound after confirming the material compatibility. Operator errors were found when a new water engineer took over the duty from his predecessor. It was also found that there were some deficiencies in the standard operating procedures (SOPs), and that on-the-job training was not enough. The water engineer failed to disinfect the sampling port and water drainage system. The RO membrane had been used for 4 years, even though the SOP standard specified changing it as every 3 years. Various bacteria, such as Rothia mucilaginosa, were cultured from the RO water sampled from the equipment. Because Rothia mucilaginosa is a resident in the oral cavity and upper respiratory tract, it is believed that the bacteria were introduced into the system by the maintenance personnel or working environment. Therefore, the presence of R. mucilaginosa implied the failure of sanitary maintenance procedures. This study suggests that water systems should be designed based on the plans for profound system maintenance. It also suggests that SOP and on-the job training are essential to avoid any operator errors. These results must be carefully considered when either constructing new RO systems or performing maintenance and periodical examination of the equipment.
LAY ABSTRACT: Reverse osmosis (RO) water is used for final rinsing in our washer-disinfector. The authors used the Ishikawa Fishbone diagram to clarify the critical points for optimizing RO water quality. There existed no measures to prevent contamination in the heat-labile RO water system. The storage tank was significantly contaminated and had to be replaced with a new one equipped with a sampling port and water drainage system. Additional filters and an UV treatment lamp were installed. The whole system disinfection started 1.5 years later using a peracetic acid–based compound after confirming the material compatibility. Operator errors occurred when a new water engineer took over the duty from his predecessor. There were neither standard operating procedures (SOPs) nor on-the-job training. The new water engineer had failed to disinfect the sampling port and water drainage system. Rothia mucilaginosa was cultured from the RO water. It is a resident in the oral cavity and upper respiratory tract. This implied the possible failure of sanitary procedures in the system maintenance. The Ishikawa Fishbone diagram was useful for this study. It suggests that water systems should be designed with plans for system maintenance taken into account. It also suggests that SOP and on-the job training are essential in order to avoid operator errors.
Introduction
This report deals with the construction and management of a reverse osmosis (RO) water system used in the washer-disinfector for reprocessing surgical instruments. There had been some deficiencies in the facility itself and management system since it was constructed 5 years ago, and the operator had often been changed without sufficient training. The conclusion of this report is that all of the deficiencies were overcome and the management system was analysed by utilizing the Ishikawa Fishbone diagram. Based on the surveillance, the RO equipment was reconstructed, and the current management system was established.
Management of water quality is important in healthcare settings (1). For instance, hemodialysis units consume a large amount of water to produce dialysis fluid. Pure dialysis water and fluid help to improve the treatment of dialysis patients. Microbiological and chemical water quality should be monitored closely for this purpose (2). Water quality is also important for reprocessing surgical instruments. Water treatment should be performed to prolong the service life of instruments and help minimize the risk of adverse patient events resulting from contaminated instruments (3).
When water is heavily contaminated, unacceptable amount of endotoxin may remain on the instruments and predispose patients to toxic anterior segment syndrome (TASS) in cataract surgery with intraocular lens implantation (3, 4). In our situation, a single chamber washer-disinfector (WD) was installed in December 2006 along with an RO plant. The WD was used for reprocessing surgical instruments. The RO water was used for the final rinsing in the operating cycle of the WD. It follows that the quality of the RO water was monitored closely.
As a result, numerous operational problems were encountered in the 5 year period, and these include the significant contaminations of and replacement of the storage tank, the installation of additional filters and the UV treatment lamp, the periodic disinfection of whole water system, filter performance degradation, the removal of the contaminated sampling port, and various errors caused by operators. The authors dealt with these problems in a reactive management style.
Incidentally, cause and effect analysis has been proven successful in improving the quality of water in the manufacturing industry. The method uses the Ishikawa Fishbone diagram to identify the root causes of a problem (5).
This study analyzed our experience in the management of an RO water system utilizing the Ishikawa Fishbone diagram. The results lead to the promotion of preventive system management for RO water. These results must be carefully considered when either constructing new RO systems or performing maintenance and periodical examination of the equipment. It seems likely that studying of the process is also essential in the management of water system for parenteral drugs.
Materials and Methods
1. Single-chamber WD with RO Plant
Our single-chamber WD (G 7836 CD, Miele, Gütersloh, Germany) with an RO plant (TRO-150A, TOYOBO Engineering Co. Ltd., Osaka, Japan) was installed in December 2006. A vendor (Muranaka Medical Instruments Co., Ltd., Tokyo, Japan) delivered the WD to our surgical center from the importer of WDs (Miele Japan Co., Ltd., Tokyo, Japan). The same vendor delivered the RO plant from the manufacturer.
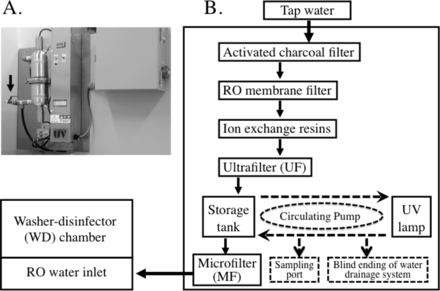
The operating cycle of the WD consisted of pre-wash, washing, intermediate rinsing, final rinsing with heated disinfection, and drying. Sixteen liters of RO water was used for the final rinsing in each operating cycle. RO water was produced from tap water and stored in the 100 L tank of the RO plant. The RO water was supplied from the storage tank to the chamber of the WD. The RO plant manufacturer was responsible for the maintenance from the RO plant to the water inlet of the WD. The WD manufacturer was responsible for the process of maintenance from the water inlet to the chamber (Figure 1). The water inlet failed to withstand heat over 70 °C.
A. The arrow indicates the sampling port of new storage tank. B. The figure explains schematically the RO water system remodeled in February 2007. The new storage tank was equipped with a sampling port and water drainage system.
2. Test Methods To Evaluate Water Quality
Endotoxin and heterotrophic plate count were measured for the microbiological water quality. Endotoxin was determined through the limulus amoebocyte lysate test, specific for endotoxin, with a sensitivity of 0.001 EU/mL (BioMedical Laboratories, Tokyo, Japan). Heterotrophic plate count was determined through the membrane filtration method using a 100 mL water sample for each filtration (6). The RO water sampled from the equipment was filtered with a 0.45 μm membrane filter and incubated at 35 °C for 48 h on soybean casein digest agar (SCDA) since December 2006.
Bacterial tests were also performed using Reasoner's 2A (R2A) agar. From March 2009 to February 2010, 0.20 μm rated membrane filters were used and the sample was incubated on the R2A agar at 30 °C for 7 days. From March 2011, 0.45 μm membrane filters were used and the sample was incubated on the R2A agar at 20 °C for 7 days in the contract laboratory (BioMedical Laboratories). The bacterial species were identified either by polymerase chain reaction amplification and sequencing analyses of 16S rRNA gene or by the Vitek 1 system (Sysmex-bioMerieux, Tokyo, Japan) (6).
When the sampling port was removed from the storage tank, the lumen was swabbed for the microbiological examination. The sample was cultured on SCDA at 35 °C for 24 h and on R2A agar at 20 °C for 7 days. The bacterial species were identified by the Vitek 1 system in the contract laboratory (BioMedical Laboratories).
Conductivity, pH, and total water hardness were measured to determine the chemical quality of the RO water (6). Chloride and iron concentrations were also measured (Nishinihon Engineering Consultant Ltd., Shiga, Japan).
3. Periodic Maintenance and Chemical Disinfection of the RO Water System
The RO plant contained some expendable parts (Figure 1). Periodic replacement had been recommended as follows: activated charcoal filter and iron exchange resins every 6 months, microfilter and UV lamp every year, ultrafilter every 1.5 years, and RO membrane every 3 years. The expendable parts were replaced, and the whole water system was disinfected every 6 months starting in August 2008. The technical assistant from the vendor operated the WD and assisted the water engineer.
The water engineer, technical assistant, and hospital staff all wore scrub suits, caps, and masks. Tools were disinfected using ethanol prior to use. Hospital staff have been supervising since March 2012, ensuring that the water engineer and technical assistant always practice sanitary procedures while performing system maintenance.
For chemical disinfection, the RO plant manufacturer offered the compound (KINOSAN PA-400, Clean Chemical CO., ltd. Osaka, Japan) containing peracetic acid (PAA) (4–4.5%), hydrogen peroxide (<6%), and acetic acid (40%). A 100 to 200 fold dilution and disinfection time over 60 min were recommended for the practical use of this compound. It was used after the material compatibility in the WD was confirmed.
The original compound was poured into the storage tank and diluted with RO water. The water engineer determined the dilution rate and disinfection time based on the latest microbiological water quality. The chamber of the WD as well as the sampling port and water drainage system were filled with the diluted compound for chemical disinfection. The diluted compound was also re-circulated in the UV radiation loop.
It was found that the microfilter failed to withstand pH below 4.0. The water engineer dislodged the microfilter temporarily from the water system during chemical disinfection. Rinsing was performed thoroughly in the whole water system while conductivity, pH, and the concentration of residual hydrogen peroxide in the chamber of WD were being monitored (6). The water engineer restored the microfilter, either new or used, to the water system after confirming that the pH was over 4.0 in the chamber.
4. Management of the RO Water System and Monitoring Water Quality
The quality of the final rinse RO water was evaluated in the storage tank for RO water as well as in the chamber of the WD according to Association for the Advancement of Medical Instrumentation (AAMI) AMMI TIR 34: 2007 (3). Hospital staff sampled water aseptically from the storage tank. They also sampled the final rinse RO water aseptically from the unloaded chamber (6).
Water quality was evaluated three times when the WD and RO plant were installed: in December 2006, in January 2007, and after remodeling the RO plant in February 2007. Evaluation of water quality was resumed in August 2008 when the periodic maintenance and disinfection of the whole water system was initiated. Water samples were taken before and after each maintenance and disinfection (Tables I and II).
RO Water Quality in the Storage Tank
A. Microbiological & Chemical Quality
B. Bacterial Species Isolated Using R2A Agar
RO Water Quality in the Chamber of the WD
A. Microbiological & Chemical Quality
B. Bacterial Species Isolated Using R2A Agar
The following aspects were also taken into account when monitoring water quality: design and remodeling of the RO water system, maintenance and chemical disinfection procedures, presence or absence of operator errors, replacement of expendable components, and so on. Data such as water quality, the replaced expendable parts, the dilution rate of a PAA-based compound, disinfection time, and so forth were documented and reported to hospital staff. Whenever hospital staff detected deterioration in the quality of the RO water, the operator-in-charge was consulted and advised to identify and rectify the causes for deterioration.
Results
1. Management of Water Quality in the Storage Tank for RO Water
Microbiological and chemical quality monitored in the storage tank for RO water is shown in Table IA. Microbial colony counts are shown in Table IB, classified according to the identified bacterial species.
Endotoxin levels ranged from 0.013 to 0.191 EU/mL. Water hardness was 0 ppm CaCO3, and iron concentrations were <0.01 mg/L. These values satisfied AMMI TIR 34: 2007, which requires following numerical limits: <10 EU/mL for endotoxin, <1.0 ppm CaCO3 for water hardness, and <0.2 mg/L for iron concentration (Table III).
Guidelines on the Quality of Pure Water
Conductivities ranged from 0.54 to 11.0 μS/cm. The conductivity satisfied the numerical limit of AMMI TIR 34: 2007 (<1.0 μS/cm) only four times. Chloride concentrations ranged from 0 to 0.6 mg/L. The results satisfied the numerical limit of the guideline (<0.2 mg/L) only twice (Table III).
Colony counts increased to 2.5 × 103 CFU/100 mL in January 2007 on the SCDA incubated at 35 °C for 48 h. The result exceeded the numerical limit of 103 CFU/100 mL in AMMI TIR 34: 2007 (Table III). It was found later that this was caused by the formation of biofilms in the storage tank.
The contaminated storage tank was replaced with a new one in February 2007. The new, heat-labile, plastic tank was equipped with a recirculation loop for continuous-flow UV radiation. The UV lamp, contained in a transparent quartz sleeve, emits UV light at a wavelength of 254 nm with the radiant energy dose of 30 milliwatt-s/cm2. The new tank was equipped with a sampling port 139 mm in length with an internal diameter of 18 mm. Blind ending was also present in the water drainage system of the new tank, which was 140 mm in length with an internal diameter of 20 mm. Moreover, ultrafilter (pore size 0.01 μm, nominal molecular weight cut-off of 300,000) was added to the upstream of the storage tank for RO water. Finally, chemical disinfection was performed from the new tank to the inlet of the microfilter using sodium hypochlorite at 20 ppm for 1 h (Figure 1).
Immediately after remodeling the RO plant in February 2007, colony counts decreased to 6 CFU/100 mL. Periodic whole-system disinfection using a PAA-based compound was initiated 1.5 years later. As a result of this delay, colony counts increased to188 CFU/100 mL in August 2008. After the initiation of whole-system disinfection, colony counts were confined to the level of 0 to 2 CFU/100 mL until August 2010. These colony counts were determined on the SCDA incubated at 35 °C for 48 h.
A new operator for water engineering took over the duty from his predecessor in March 2011. On this occasion, there were also inadequate standard operating procedures (SOPs), and on-the-job training was not given. The new water engineer performed his duty following only verbal directions, and failed to disinfect the sampling port.
In the period from March 2011 to March 2012, colony counts reached a maximum of 21 CFU/100 mL at the existing sampling port. After the port was removed, colony counts decreased to 1 CFU/100 mL in July 2012. These colony counts were determined on the SCDA incubated at 35 °C for 48 h. The slime was swabbed from the removed port and yielded 800 colonies of Ralstonia picketti on the SCDA incubated at 35 °C for 24 h. R. picketti also grew on the R2A agar incubated at 20 °C for 7 days.
When the sampling port was removed in March 2012, hospital staff discontinued sampling the RO water from the storage tank at the end of water system disinfection. This was because the sampling procedure itself might cause contamination of the storage tank.
Colony counts were also determined on the R2A agar incubated at 30 °C for 7 days from March 2009 to February 2010. Colony counts ranged from 5 to 301 CFU/100 mL during this period. After March 2011, R2A agar was incubated at 20 °C for 7 days. Colony counts ranged from 93 to 393 CFU/100 mL until March 2012.
In July 2012, colony counts again increased to 3.6 × 103 CFU/100 mL immediately prior to the periodic water system maintenance. Unidentifiable Gram-negative rods were the main isolate (Table IB). The contract laboratory reported these results 10 days after the end of periodic water system maintenance. Then, it came to light that the new water engineer had again failed to disinfect the water drainage system after taking over the duty. The water engineer consented to the preparation of SOPS and performance of on-the-job training for system maintenance.
2. Management of RO Water Quality for Final Rinsing in the WD chamber
The quality of final rinse RO water monitored in the WD chamber is shown in Table IIA. Microbial colony counts are shown in Table IIB, classified according to the identified bacterial species. Endotoxin levels ranged from 0.116 to 1.121 EU/mL. Water hardness values were 0 ppm CaCO3, and iron concentrations were less than 0.01 mg/L. These values satisfied the numerical limits of AMMI TIR 34: 2007 (Table III).
Conductivities ranged from 1.0 to 54.7 μS/cm. It was noticed that the RO membrane had been used over 4 years when conductivity increased to 54.7 μS/cm in March 2011. Chloride concentrations ranged from 0.2 to 2.6 mg/L. Conductivities and chloride concentrations failed to satisfy the numerical limits of AMMI TIR 34: 2007 (Table III).
Colony counts ranged from 2.3 × 103 to 2.5 × 103 CFU/100 mL in January and February 2007, and further increased to 6.0 × 103CFU/100 mL in August 2008. Immediately after the whole-system disinfection in August 2008, colony counts decreased to 188 CFU/100 mL. After March 2009, colony counts were confined to the level of 0 to15 CFU/100 mL, which was considered as a normal level for RO water. These colony counts were determined on the SCDA incubated at 35 °C for 48 h.
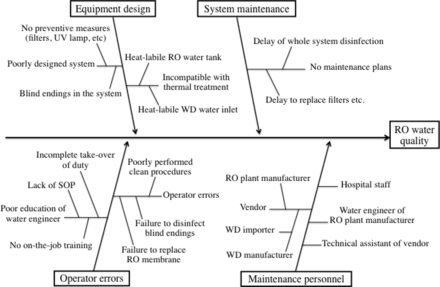
A PAA-based compound was used after the material compatibility in the WD was confirmed. This confirmation took 1.5 years to be completed. It was revealed that the process manufacturer designed the WD such that it is compatible with the practical use of our PAA-based compound when used with RO water. The lack of smooth communication with the vendor, importer, and manufacturer of the WD was found to be a major cause of this problem (Figure 2).
The Ishikawa Fishbone diagram shows the root causes responsible for the deteriorated RO water quality.
Colony counts were also determined on the R2A agar incubated at 30 °C for 7 days from March 2009 to February 2010. Colony counts ranged from 21 to 1.1 × 104 CFU/100 mL during this period. Colony counts again exceeded the numerical limit of 103 CFU/100 mL in AMMI TIR 34: 2007 twice: in March 2009 and July 2009 (Table II). Nontuberculous mycobacteria and Sphingomonas sp. were the main isolates in March 2009. Herbaspirillum sp. and Sphingomonas sp. were the main isolates in July 2009 (Table IIB).
After March 2011, R2A agar was incubated at 20 °C for 7 days. Colony counts still ranged from 21 to 1.9 × 103 CFU/100 mL. Colony counts exceeded the numerical limit of AMMI TIR 34: 2007 twice: in July 2011 and July 2012. Unidentifiable Gram-negative rods and Sphingomonas sp. were the main isolates on both occasions (Table IIB).
Discussion
The quality of final rinse water is important for the reprocessing of medical devices. Final rinse water should be of potable quality or better in ISO 15883 for WDs (7). The Instrument Preparation Working Group recommends fully de-mineralized water for final rinsing, which satisfies the quality for boiler feed water as defined in EN 285 (Table III) (8).
Under these circumstances, AAMI TIR 34: 2007 defined the quality of RO water for reprocessing medical devices (Table III). The guideline also recommends RO water for final rinsing of devices, which are sterilized by steam or low-temperature gas (3).
RO water is vulnerable to contamination (9). Once water-borne bacteria form biofilms, bacterial eradication becomes extremely difficult. It follows that a preventive management style should play a key role in the quality control of RO water (3, 10). Contrary to this expectation, the quality control of final rinse water had been performed in a reactive management style (11). This study analyzes our experience in the management of our RO water system, utilizing the Ishikawa Fishbone diagram, to promote the preventive system management for RO water (12) (Figure 2).
The design of the water system is closely associated with the system maintenance. Final rinse RO water should be stored in the tank at no less than 65 °C, according to ISO 15883 for WDs. The temperature may decrease in the tank when it is replenished with incoming cold water. For this reason, the guideline recommends a higher temperature such as 75 °C in the tank (7). This method disinfects the whole water system thermally in each operating cycle of the WD.
On the other hand, the RO water system contained heat-labile components: the plastic storage tank and water inlet of WD. They were unlikely to withstand the thermal treatment required in ISO 15883 for WDs (7). Some alternative methods should have been instituted in our RO water system to compensate for the inability to withstand thermal treatment.
However, there existed no measures to prevent contamination in the heat-labile RO water system. As a result, the storage tank was significantly contaminated and was replaced with a new one equipped with a sampling port and water drainage system. Additional filters and a UV treatment lamp were also installed. These procedures improved colony counts in the new storage tank.
In this context, the periodic disinfection of the whole water system should also have started immediately after remodeling the RO water system. However, the initiation of the whole-system disinfection was delayed by 1.5 years. It seems likely that the delay was due to the poor communication among the vendor, importer, and manufacturer of the WD. This failure sheds light on a critical aspect in the system maintenance where numerous personnel are involved (Figure 2). The Ishikawa Fishbone diagram might have been useful in overcoming this problem because the diagram helps the whole team to reach a consensus swiftly (5).
Numerous operator errors occurred in March 2011 when a new water engineer took over the duty from his predecessor. There were neither appropriate SOPs nor on-the-job training. The RO membrane had been used over 4 years although it should be replaced every 3 years. The conductivity of RO water increased to 54.7 μS/cm, which was far over the empirical threshold of 15 μS/cm in EN 285 (Table III). Moreover, the new water engineer failed to disinfect the sampling port and water drainage system. These operator errors predisposed the system to deteriorated RO water quality. It seems likely that these errors could have been prevented if SOPs and on-the-job training had been available for system management.
Heterotrophic plate count is an important process indicator of microbiological water quality (3). Incubation by using different kinds of media reveals that each medium has a different ability to grow bacterial colonies. If a poor culture method is selected, the state of contamination will probably be underestimated (2).
The water engineer employed the culture method using SCDA incubated at 35 °C for 48 h. This method is the culture condition for nutrient-enriched bacteria, and hence is unsuitable for water-borne bacteria in the nutrient-poor environment. Moreover, the method seems to be unsuitable for culturing slow-growing bacterial species (2, 13).
To increase the ability to grow heterotrophic bacterial colonies, nutrient-poor R2A agar was used and incubated for 7 days at 30 °C from March 2009 (14). To further increase the ability, the incubation temperature was lowered to 20 °C in March 2011 (2). This method revealed 3.6 × 103 CFU/100 mL in the storage tank in July 2012. Then, the presence of contaminated water drainage system came to light, which arose from the operator's error.
As for the microbiological examination of the slime taken from the sampling port, as many as 800 colonies of R. picketti were cultured on SCDA incubated at 35 °C for 24 h. Immediately before the removal of sampling port, the RO water yielded 10 CFU/100 mL in the storage tank on the SCDA incubated at 35 °C for 48 h. Five months after the removal of sampling port, colony counts decreased to 1 CFU/100 mL using the same culture method. It appears that a biofilm of R. picketti had formed in the sampling port, contaminating the RO water in the storage tank (15).
Various bacteria were cultured from RO water, which included nontuberculous mycobacteria, Methylobacterium sp., Sphingomonas sp., Ralstonia sp., Herbaspirillum sp., Paenibacillus sp., and Rothia mucilaginosa (Tables IB, IIB). They are opportunistic pathogens derived from the environmental reservoir such as water or soil except for R. mucilaginosa (6, 16⇓⇓–19).
R. mucilaginosa is a Gram-positive coccus and a resident in the oral cavity and upper respiratory tract (17). It follows that the source of R. mucilaginosa should be of human origin: most likely directly from maintenance personnel, or indirectly from the working environment via contaminated hands. Therefore, hospital staff have been supervising closely since March 2012 to ensure that maintenance personnel perform sanitary procedures such as wearing a mask and clean or sterile gloves.
Herbaspirillum sp. are Gram-negative, motile, nitrogen-fixing bacteria (18). Paenibacillus sp. are Gram-variant or -negative, spore-forming, motile rods. A novel nitrogen-fixing type is also reported (19). Nitrogen fixation is a beneficial process for root-colonizing bacteria to form symbiotic relationships with plants (18, 19). It seems likely that these nitrogen-fixing soil bacteria might have derived from the working environment such as the floor or the packaging system of expendable parts via poorly disinfected tools, failure to ensure the clean zone for sanitary procedures, and the reuse of contaminated microfilters.
Bacteria are able to increase resistance to chemical disinfectants. For instance, Tschudin-Sutter and co-workers reported glutaraldehyde-resistant Pseudomonas aeruginosa (20). Therefore, it is important to always bear in mind that some bacterial species may develop resistance to a PAA-based compound.
The Ishikawa Fishbone diagram helped to classify the factors responsible for the deteriorated RO water quality (12, 21) (Figure 2). The upper half of the diagram shows the equipment-related causes: poorly designed system and lack of maintenance plans. The lower half shows the causes concerned with human factors: operator errors and numerous personnel involved in the system maintenance.
Conclusions
The Ishikawa Fishbone diagram was useful in revealing the critical points for improving the quality of RO water. It seems likely that these findings would pave the way for the preventive system management for RO water. The present analysis suggests that RO water systems should be designed with plans for profound and careful system maintenance taken into account. Microbial tests should be performed by using various kinds of culture media if the water is suspected to be contaminated. The study also suggests that SOPs and on-the job training are essential in order to avoid operator errors.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgements
The authors express sincere thanks to Dr. Winfried Michels for his kind instruction and cooperation. The authors also express sincere thanks to Mr. Takayuki Ohta, Mr. Takayuki Hirayama, and Mr. Yasuo Sugimoto for their kind cooperation.
- © PDA, Inc. 2013