Abstract
Storing protein formulations in the frozen state typically improves stability during long-term storage as a drug substance or as a drug product. The frozen state minimizes chemical degradation and physical instability. However, the frozen state is not an optimal storage condition for the glass vial itself. A significant issue was observed when small, flake-like pieces of glass particles (lamellae) appeared in vials containing thawed protein product. The occurrence of glass particles during freeze-thaw results in product rejection and potentially, adverse events. In recent years, glass flakes due to chemical delamination have been observed in parenteral liquid formulations after long-term storage, resulting in a number of product recalls. In this study, for the first time, glass delamination is reported in pharmaceutical glass vials containing frozen protein formulation, caused by a novel mechanism involving thermally-induced mechanical stress.
In this article, a monoclonal antibody drug product in glass vials and the corresponding placebo vials were studied to identify the contributing factors from the freeze-thaw process, such as freezing temperature, the presence or absence of protein, and other handling conditions. Freezing temperature was found to be the most critical factor. Glass lamellae were only observed when the products were frozen to −70 °C, while freezing only to −30 °C did not cause any lamellae formation even after multiple freeze-thaw cycles. Protein concentration and the handling of the vials were also identified as contributing factors. A concentration gradient which formed after freeze-thaw induced a higher rate of lamellae occurrence in a subsequent freeze-thaw cycle compared to vials without the concentration gradient. Analyses by Fourier transform infrared spectroscopy and scanning electron microscopy/energy dispersive spectroscopy confirmed that the flake-like lamellae were thin, flat glass particles. Defects corresponding to the glass flakes were observed by scanning electron microscopy on the inner surface of the vials that contained lamellae. In addition, inductively coupled plasma mass spectrometry testing did not show elevated levels of silicon in the drug product solution, suggesting that the glass lamellae formed in the frozen vials was a local, event-based phenomenon rather than silica dissolution from the product contact surface or glass degradation caused by corrosive attack. These findings can be explained by the same thermally-induced mechanical stress which caused vial breakage. Frozen protein formulations contracted below −30 °C, causing an inward glass deformation and a subsequent rapid movement of the glass when the frozen plug of drug product solution separated from the vial inner surface at approximately −50 to −60 °C. The mechanical stress released during this separation caused vial breakage. The incidence of vial breakage increased with more concentrated product and higher fill volume–to–vial volume ratios. The same mechanism applies to lamellae formation. As the rapid surface separation occurred, small, thin pieces of glass were pulled from the glass surface by the frozen plug, and, as a result, glass lamellae particles appeared in the drug product solution after thawing.
LAY ABSTRACT: In recent years, glass flakes have been observed in parenteral liquid formulations due to chemical delamination during long-term storage, resulting in a number of product recalls. In our study, we discovered a novel mechanism of glass delamination in vials containing frozen protein formulations. This glass delamination mechanism has never been reported before, and we believe this work will benefit the pharmaceutical scientific community, especially the biotechnology and parenteral drug industries.
Storing protein formulations in the frozen state typically improves stability during long-term storage as a drug substance or as a drug product. The frozen state minimizes chemical degradation and physical instability. However, the frozen state is not an optimal storage condition for the glass vial itself. In this study, we observed that after thawing, small, flake-like pieces of glass particles (i.e., lamellae) appeared in vials containing frozen protein formulation. To investigate the root cause, we performed a series of freeze-thaw experiments and characterized the lamellae particles, the vial inner surface, and the elemental composition of the solution. The root cause was determined to be mechanical stress caused by thermal contraction of frozen protein formulations below −30 °C. This contraction caused an inward glass deformation on the vial sidewall and, subsequently, the glass vial surface abruptly separated from frozen protein formulation. Under this mechanical stress, small, thin glass pieces were peeled from the vial inner surface by the frozen formulation, causing lamellae formation. The experimental design and results leading to the discovery of the novel glass delamination mechanism are presented in detail in this article.
Introduction
The presence of visible foreign particulates in injectable drug products is unacceptable. Glass particles have been observed in injectable protein therapeutics as well as other parenterals filled in glass vials, resulting in product recalls of dexamethasone sodium phosphate, concentrated sodium chloride, methotrexate, Epogen/Procrit, and fluorouracil injections, among others (1, 2). The potential risks have drawn significant attention from both the pharmaceutical industry and the regulatory agencies because of potential adverse effects such as embolic events and unwanted adjuvant effects resulting in increased immunogenicity of therapeutic proteins (3).
Glass delamination has been well described in the literature within material and pharmaceutical sciences, and most reported events were caused by glass degradation from the corrosive attack of hydroxide ions on the glass matrix (4⇓⇓⇓–8). The hydroxide ion can penetrate the glass surface to a sufficient depth to initiate significant attack on the silicate structure. As the silica matrix dissolves, the glass interface will degrade, causing delamination of the glass surface (9, 10). Alkalinity testing serves as the primary tool for evaluation of glass surface hydrolytic resistance, and the test is included in the European Pharmacopeia (Ph.Eur.) and the International Organization for Standardization (ISO) guideline (11, 12). Besides the glass chemistry and formulation pH (13⇓–15), the presence of certain pharmaceutical compounds (10), citrate and tartrate buffers (16), and potassium chloride salt (13) can affect glass dissolution and delamination. In addition, pharmaceutical manufacturing processes such as terminal sterilization by autoclaving (8, 13, 17⇓–19) have also been reported to accelerate glass delamination. Delamination requires time, and flaking may become visible only after long-term storage as chemical erosion progresses (13, 20). Dissolution of silica is considered an effective indicator that delamination is occurring (14).
Frozen storage conditions are often used to retain protein stability for labile biopharmaceutical drug products, particularly for the drug substance or drug product in the early development stage for molecules with limited formulation stability data. Recently, thin, flake-like glass particles (lamellae) were observed in stability samples of multiple frozen early-stage protein products. The occurrence and severity of the glass lamellae in thawed vials did not show any correlation to the product age, and lamellae were observed in some of the vials within a month after filling. None of these protein products was formulated at neutral or higher pH, or was formulated with a citrate or tartrate buffer. This phenomenon cannot be explained by the known mechanism of glass delamination attributed to corrosive attack; it appears to be caused by a different mechanism associated with freeze-thaw.
When investigating the product temperature exposure history, it was found that the vials which contained lamellae all experienced temperature conditions around −70 °C during shipping on dry ice or during interim staging prior to stability testing. In 2007, we published findings from an investigation study of glass vial breakage observed with frozen protein drug product vials (21). The primary cause for vial breakage was the thermal contraction of protein solutions below −30 °C causing inward deformation of glass on the sidewall and the subsequent release of this mechanical stress when the vial wall abruptly separates from frozen liquid. Such mechanical stress caused cracks on the sidewall a few millimeters from the vial bottom. Based on the vial breakage study results and the temperature exposure history for lamellae-containing vials, we postulated that the formation of glass lamellae could be related to the same mechanism causing vial breakage. The leading hypothesis is that the abrupt separation of frozen protein formulation from the glass vial inner surface can peel off small, thin glass pieces from the vials, causing lamellae formation during −70 °C exposure. A series of studies was conducted to test this hypothesis, and the results are discussed in detail below.
Materials and Methods
Materials
Drug product vials containing a monoclonal antibody (Antibody A) and corresponding placebo vials were utilized in the study. The composition of placebo is the same as drug product except for the protein. To minimize particles introduced by filling process and also to avoid any artifacts introduced by lot-to-lot vial differences, all vials in this study were filled in a class 100 clean room of a good manufacturing practice (GMP) fill-finish facility using the same batch of 10 cc type 1A borosilicate tubing glass vials manufactured by Nipro Glass Americas (Westport, IN) with alkalinity ranging from 61% to 77% per the Ph.Eur. hydrolytic resistance test. The two fills occurred within a 2-week time frame for the drug product and placebo, respectively, to minimize the age difference between the materials. The 13 mm rubber stoppers and seals were purchased from Daikyo Seiko (Tokyo, Japan) and West Pharmaceutical Services (Clearwater, FL), respectively. All primary packaging components met USP and Ph.Eur. requirements. The antibody was formulated at 30 mg/mL with a formulation buffer containing stabilizer and buffering reagent (not citrate or tartrate buffer) at pH 5.2. The fill volume (including the deliverable volume and overfill) of 3.5 mL was the same for the antibody drug product vials and the placebo vials.
Pre-Inspection
Prior to the freeze-thaw study, all antibody product vials and placebo vials were visually inspected by certified inspectors. These inspectors are certified for USP/Ph.Eur./Japanese Pharmacopeia (JP) visual inspection testing and also for glass lamellae inspection per GMP procedure. Any vials containing visible particles were removed. The purpose of the pre-inspection was to ensure that only vials free of particles were used in the study to avoid any potential interference with glass lamellae inspection conducted as part of the study.
Freeze-Thaw Study and Visual Lamellae Inspection
A total of eight study arms were included to evaluate lamellae occurrences under different freeze-thaw and handling conditions (Table I). Each arm contained 315 vials, in order to ensure that a lamellae occurrence rate as low as 0.7% could be detected with 95% confidence. Each freezing or thawing step continued for at least 24 h to ensure a complete phase change. The rationale for the chosen freeze-thaw conditions is described below:
Arms A through H used antibody vials. Arms A, B, C, and D represent a factorial design for two different freezing temperatures (−30 °C vs. −70 °C) and two different handling conditions (inspection vs. no inspection between the first and second freeze-thaw cycles: mixing occurs during inspection). Arm E incorporates a hybrid of the first freeze-thaw at −30 °C, followed by a second freeze-thaw at −70 °C with no inspection in between. Arm F simulates a typical distribution condition: Vials are frozen at −30 °C in the warehouse, and later shipped on dry ice (approximately −70 °C) to the clinical sites. The vials are thawed at 2–8 °C before administration.
Arm G and H used placebo vials. Arm G represents one extreme of the bracketing conditions with both freeze-thaw cycles at −30 °C and inspection in between; and arm H represents the other extreme of the bracket conditions, with both freeze-thaw cycles at −70 °C and no inspection in between. Arm G contrasts with Arm A (with and without protein, same freeze-thaw cycles and inspection). Similarly, Arm H contrasts with Arm D. From these results, we can determine the impact of the presence of protein in the formulation.
Summary of the Design of the Freeze-Thaw Experiments: Freeze-Thaw Temperature Conditions and Inspection Requirements of the Drug Product and Placebo Samples
A total of 1890 antibody vials were randomly divided into study Arms A through F, and 630 placebo vials were randomly divided into study Arms G and H (315 vials in each arm, see Table I). The vials were packed into corrugated cardboard paper boxes (each arm had 4 boxes, 80 vials in the first 3 boxes and 75 vials in the 4th box) with appropriate labeling on the boxes.
The certified inspectors performed the visual inspections after the first thaw for Arms A, C, and G only. As part of the procedure for visual inspection, the inspector swirls or inverts the vial to increase the visibility of particles. Swirling or inverting the vial causes the vial contents to be mixed. During inspection, lamellae-containing vials were segregated, and only lamellae-free vials proceeded to the second freeze-thaw cycle. After the second thaw, inspection was performed on vials from all arms, A through H.
Statistical Analysis on Lamellae Occurrences
Statistical analysis was performed using JMP v8.0 by SAS Institute. To facilitate the analysis, combinations of temperatures in the first and second freeze (such as −30 °C/−30 °C, −30°C/−70 °C, −70 °C/−70 °C) were treated as a categorical variable named “Group”. The variables of interest included Group, Inspection (with and without the first inspection), and Product (yes for antibody and no for placebo). Comparisons were performed across various tested conditions using Fisher's exact test in cases of two-group comparisons and Chi-square test when more than two groups are compared. Under both tests, the null hypothesis (H0) was that the proportion of lamellae-containing vials in each group was the same, and the alternative hypothesis (H1) was that it was not. The null hypothesis was rejected in favor of the alternative hypothesis and proportion differences were deemed statistically significant if the test p-value was less than 0.05.
Characterizations of Lamellae Particles and Vial Surface
The contents of representative lamellae vials were filtered through 0.8 μm (pore size) gold-coated polycarbonate membranes (filtr.AID RGG1010008_e, Rap ID, Berlin, Germany) to retain the lamellae (one vial per filter). The filter was further rinsed by high-performance liquid chromatography grade water to remove soluble substance. The filters were then examined under the Zeiss Stemi 2000C Optical Microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY), and color images of the particles were captured using an AxioCam MRC color digital camera attached to the microscope.
Large lamellae particles were further examined under Fourier transform infrared (FTIR) spectroscopy and scanning electron microscopy/energy dispersive spectroscopy (SEM/EDS) for identification. FTIR analysis was performed with a Bruker Hyperion 3000 Microscope attached to a Tensor 27 FTIR spectrometer (Bruker Optics, Billerica, MA). The FTIR spectra were obtained in reflection mode with the particles on the gold-coated filters. The FTIR spectra were collected for 128 scans with a 4 cm−1 spectral resolution. For the SEM/EDS analysis of the filtered particles, the gold-coated filters were directly placed on the sample stage and secured with a double-sided copper adhesive tape. The Zeiss EVO MA10 SEM (Carl Zeiss Microscopy, LLC) was utilized to image the samples by secondary electron detector using the variable pressure mode and an accelerating voltage of 20 kV. The Oxford INCA Energy 250 EDS (Oxford Instruments, Elk Grove Village, IL) attached to the SEM was used for the elemental analysis.
The inner surface of a representative lamellae-containing vial was examined with SEM for surface defects. Strips were cut from the vial by a diamond saw and rinsed with Millipore deionized water. The Zeiss EVO MA-10 SEM was used in the high-pressure mode to image the sample with a variable pressure secondary electron detector and an accelerating voltage of 20 kV.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
ICP-MS analysis was performed on vials from all freeze-thaw arms (A through H), along with antibody and placebo control vials which were kept at 2–8 °C without freeze-thaw. The ICP-MS (Perkin Elmer, Elan DRC II) was optimized following the standard procedures. Samples were scanned for inorganic elements in standard and dynamic reaction cell (DRC) modes. The standard mode is for general scanning of elements of m/z ranges of 6–15, 19–39, 42–210, and 230–240. A 50 ppb standard containing 43 elements (BDH, 820026-108) served as an external standard. Each sample was diluted 60-fold with deionized water (6 mL total volume) and analyzed for type and concentration. The DRC mode detects potassium (K), calcium (Ca), chromium (Cr), iron (Fe), and zinc (Zn), which are difficult to analyze in standard mode because of the instrument's naturally occurring polyatomic interferences. Separate calibration curves for K, Ca, Cr, Fe, and Zn were prepared with the internal standard, cobalt (Co). Each sample was diluted 30-fold with 0.5% nitric acid, spiked with 50 ppb Co, and analyzed. The acquired response was converted to the concentration using the standard curve.
Results and Discussion
Lamellae Occurrences during Freeze-Thaw
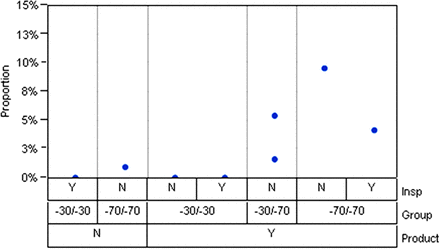
No vial breakage was observed in the freeze-thaw experiment. The lamellae occurrences from the first and second inspections are included in Table II. To further facilitate the statistical analysis, combinations of temperatures in the two freezing steps (such as −30 °C/−30 °C, −30 °C/−70 °C, −70 °C/−70 °C) were treated as an additional categorical parameter named “Group”. Figure 1 shows the lamellae occurrence rate (%) under three parameters (Group refers to freezing temperature combinations; Insp refers to the first inspection – yes (Y) or no (N), mixing occurred during the inspection; and Product refers to – Y for the antibody drug product or N for placebo). Three key contributing factors can be identified below from the lamellae results:
Visual Inspection Results from the Freeze-Thaw Experiments: Number of Vials Containing Lamellae Observed during the 1st and 2nd Inspections, and the Cumulative Counts. N/A - Not Applicable Because the 1st Inspection Was Not Performed
Dependence of lamellae occurrence rate on three variables tested in the freeze-thaw study: Group (temperature combinations of the 1st and 2nd freeze-thaw), Insp (first inspection, Y or N), and Product (Antibody product -Y, Placebo-N).
1. Freezing Temperature Impact:
No lamellae were observed in the −30 °C freeze arms (A, B, G) even with multiple freeze-thaw cycles. In contrast, all arms that experienced −70 °C had lamellae occurrences (C, D, E, F, H). Arms A and C (0 vs. 13 lamellae-containing vials), and Arms B and D (0 vs. 30 lamellae-containing vials), in which the only difference is the freezing temperature (−30 °C freeze for A and B; −70 °C freezing for C and D), constitute direct comparisons of the effect of freezing temperatures. The statistical analysis on Arms B, D, and E further confirmed that the temperature exposure induced differences in lamellae occurrences across three Groups (−30 °C/−30 °C, −30 °C/−70 °C, −70 °C/−70 °C) were statistically significant (Chi-square p-value < 0.0001).
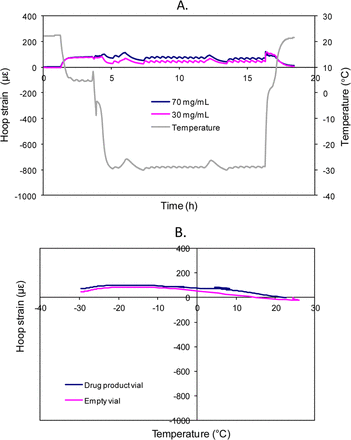
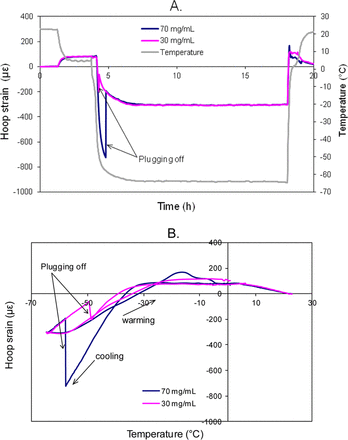
The above visual inspection results regarding lamellae occurrence support the hypothesis that lamellae formation results from the same mechanism of mechanical stress that causes vial breakage (21). To facilitate the understanding, the strain gage data are included in Figures 2 and 3. These previously reported (21) strain gage results were acquired using a different antibody (Antibody B) product formulated at 30 mg/mL and 70 mg/mL. In the strain gage experiments, there was minimal strain during the −30 °C freeze-thaw (Figure 2), and no visible surface damage or breakage of the vials occurred. In the −70 °C freeze-thaw cycle, when the product temperature decreased from −30 °C to −70 °C, the frozen product started to contract and imposed a negative (i.e., compressive) strain on the vial because the coefficient of expansion (COE) of the frozen protein formulation is higher than that of glass (COE of Type 1A glass is ∼3.3 ppm/°C). As product temperature approaches approximately −50 °C to −60 °C, the negative strain suddenly bounced back because the solidified frozen formulation abruptly separated from the inner vial surface and the contraction force was relieved, allowing the glass to relax back to its own shape under those temperatures (Figure 3). This phenomenon was described as plugging-off in our previous publication (21). When such high potential contraction energy converts to rapid movement of the glass, the hypothesis is that the frozen protein formulation peels off small, thin glass pieces from the vial surface, causing lamellae particles to appear in the drug product solution after thawing.
(A) Strain profiles of 30 mg/mL and 70 mg/mL antibody B products during freeze-thaw cycle to −30 °C only (3.5 mL in 10 cc vials, room temperature → −30 °C → 25 °C). (B) Comparison of strain profiles of product filled vial (70 mg/mL antibody B product, 3.5 mL in 10 cc vials) and an empty 10 cc vial during freezing to −30 °C from room temperature (21).
Strain profiles of 30 mg/mL and 70 mg/mL Antibody B products during freeze-thaw cycle to −70 °C (3.5 mL in 10 cc vials, room temperature → 2–8 °C → −70 °C→ 25 °C): (A) strain in time course, (B) strain as a function of product temperature (21).
Additional evidence was collected to prove that the plugging-off phenomenon occurred at the −70 °C freezing condition but not at −30 °C. Figure 4 shows the visual difference between two antibody product vials, one immediately taken from the −70 °C freezer (the vial on the left) and the other one from the −30 °C freezer (the vial on the right). In the −70 °C vial, small space gaps or channels resulted from plugging-off were observed in between the vial inner surface and the frozen solution. With these gaps, the surface of the frozen liquid appeared to be uneven and crackled with variations in whiteness. In contrast, the frozen solution in the −30 °C vial was in complete contact with the vial and the frozen solution appeared more smooth and uniform in white color.
Comparison of the appearance of two antibody product vials immediately taken from the −70 °C freezer (the vial on the left) and the −30 °C freezer (the vial on the right).
2. Impact of the Presence of Protein:
During the −30 °C freeze-thaw, no lamellae were found in either the antibody product or placebo vials (study Arms A, B, G). Therefore, the presence of protein does not have any discernable effect when the vials are frozen only to −30 °C. As expected, there is no statistical difference between Arms A and G (Fisher's exact p-value = 1.0000).
After the two cycles of −70 °C freeze-thaw without inspection in between, only 3 placebo vials (Arm H) formed lamellae in contrast to 30 antibody vials (Arm D). This difference is statistically significant (Fisher's exact p-value < 0.001), suggesting that the presence of protein substantially increased lamellae formation after freezing to −70 °C.
The increased lamellae occurrence seen in the protein product vials compared to placebo during the −70 °C freeze-thaw is in good agreement with the mechanism discovered from the vial breakage study. As shown in Figure 3, higher protein concentration induced a higher magnitude of contraction strain during −70 °C freezing. As a result, the vial was compressed more, and hence, an elevated level of potential energy would be released during the subsequent separation between the frozen formulation and the vial surface. The stronger contraction strain was most likely associated with increased viscosity and stronger adhesion between the product and glass surface in higher concentration formulations. The separation with the larger displacement and release of a higher level of stored potential energy increases the chances of peeling of small, thin glass pieces from the vial surface.
3. Impact of Mixing during Inspection:
After freeze-thaw, a concentration gradient forms in the container with higher concentration of protein and excipients at the bottom and lower concentration of protein and excipients at the top (22, 23). With this antibody product at 30 mg/mL in 10 cc vials, after a freeze-thaw cycle, either between 2–8 °C and −30 °C or between 2–8 °C and −70 °C, concentration gradients were experimentally confirmed. The protein concentration was found to range between 15 to 25 mg/mL at the top, versus 38 to 44 mg/mL at the bottom, of the vial. When the solution in the vial was mixed post freeze-thaw, a schlieren effect was also observed visually due to the presence of the concentration gradient. As a result, concentrated protein and excipients close to the bottom would likely cause higher mechanical stress/strain during −70 °C freezing, and lead to more lamellae formation if the solution was unmixed between freeze-thaw cycles. This hypothesis is supported by the observations that during the two cycles of −70 °C freeze-thaw, fewer cumulative counts of lamellae-containing vials were found in Arm C as compared to Arm D (13 vs. 30 vials). Arm C experienced the first inspection, so the protein and excipient concentration gradients were eliminated. Arm D was undisturbed between the first thaw and second freeze, so the protein and excipient concentration gradients were present prior to re-freezing. The difference in lamellae occurrence between Arms C and D is statistically significant (Fisher's exact p-value = 0.0107), suggesting that the mixing during the inspection procedure in between freeze-thaw cycles substantially affected lamellae formation in the −70 °C freeze-thaws. Additionally, in Arm C, with homogeneous solution, only two vials generated lamellae in the second −70 °C freeze-thaw. These results are expected because the second cycle had identical conditions as the first cycle and vials susceptible to lamellae formation would have shown lamellae formation in the first cycle.
Protein and excipient gradients exist in both Arm D and Arm E. The only difference between the two was the first freeze temperatures (D at −70 °C and E at −30 °C). D and E showed the two highest lamellae occurrences in this study (30 and 17 lamellae-containing vials in Arms D and E, respectively), with no statistically significant difference (Fisher's exact p-value = 0.0678). Therefore, regardless of the temperature of the initial freeze-thaw, the presence of the concentration gradient has a similar impact on lamellae formation in a subsequent −70 °C freeze-thaw.
The observations of mixing effect are well correlated with the concentration impact discussed above and also strongly support the hypothesis of mechanically induced glass delamination during low temperature exposure in the frozen state. Considering that protein contributes to viscosity, higher protein concentration at the bottom of the vial is expected to cause greater mechanical stress relative to the excipient gradient.
During the two cycles of −30 °C freeze-thaw, no lamellae were found regardless of the presence of the protein and excipient concentration gradients (study Arm A vs. Arm B). Therefore, the presence of protein does not have any discernable effect when the vials are frozen only to −30 °C. As expected, there is no statistical difference between Arms A and B (Fisher's exact p-value = 1.0000).
Characterization of Lamellae Particles and Vial Surface
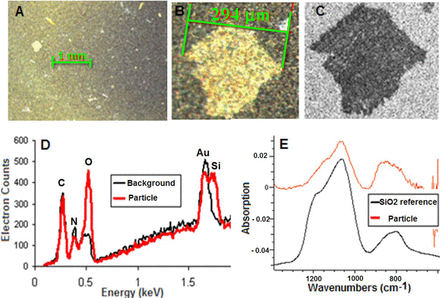
The lamellae were retained on gold-coated filters and examined by optical microscopy, SEM/EDS, and FTIR spectroscopy. The particles retained on gold filters were thin and flat under optical microscopy (OM) with a wide size distribution ranging from approximately 20 μm up to approximately 300 μm (Figure 5A). The largest lamellae particle (∼300 μm), as a representative particle, was further analyzed under OM and SEM (Figures 5B and 5C). The particle under OM appeared to be shiny and flat. The particle was then observed under SEM with secondary electron imaging. In this mode electrons are emitted from surfaces struck by the electron beam, with the counts sensitively responding to the secant of the local tilt angle (24), as a powerful tool to reveal topographic characteristics. The SEM image confirmed that the particle is flat and does not have any three-dimensional structure. The filter background is whiter in color compared to the particle because the gold created more secondary electrons compared with the particle matrix. The small black holes on the filter are the pores with sizes of 0.8 μm. The elemental analysis compared the particle area to the particle-free area. The interaction volume of the incident beam could be several microns, and signals from the background gold filter can show up in the particle area. Therefore, the particle-free area on the filter was analyzed to provide background information on the expected elements from the filter. Compared with the background signal, the particle has a much stronger oxygen signal and there is a silicon peak to the right of the gold peak (Figure 5D).
Results of lamellae particle characterization: (A) an overview of multiple lamellae particles retained on filter by optical microscopy, (B) zoom-in of a representative particle by optical microscopy, (C) SEM of the representative particle, (D) EDS of the representative particle in comparison with filter background, and (E) FTIR spectrum of the representative particle in comparison with an SiO2 reference from KnowItAll Database HSX# 5404.
The FTIR spectrum of this particle matched with the SiO2 spectrum (Figure 5E) in the Si-O stretching region. Two bands, attributed to the Si-O-Si structure, were observed in both the reference SiO2 and the particle in the region of 600–1400 cm−1. The more intense band centered at 1060 cm−1 is due to the asymmetric Si-O-Si bonds. This band usually appears between 1200 and 1000 cm−1 (25). Not only is this band very intense, but it has an asymmetric shape with a shoulder on the left hand, which might be due to the different substructures between Si-O-Si groups (26). This asymmetric shape at 1060 cm−1 could be used as a diagnostic marker for the presence of silica in a sample. Another band at 800 cm−1 is from the symmetric stretching of Si-O-Si. Based on the particle morphology and the strong silica signal (SiO2), the flake-like lamellae particles are consistent with the predominant component of glass.
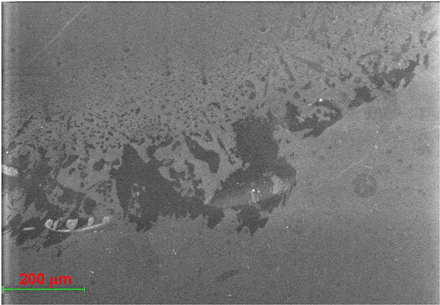
The inner surface of lamellae-containing glass vials was examined under SEM by secondary electron imaging. Surface defects near on the sidewall close to bottom of the vial showed large, discrete defects with some patches up to approximately 300 μm (Figure 6). These surface defects on the vial further supported that the thin, flat silica lamellae were peeled off from glass vial surface after the mechanical stress during freeze-thaw. Previous literature showed that when glass vials were subjected to chemical delamination by a corrosive pharmaceutical compound, inner vial surface defects were also observed by SEM, and these surface defects confirmed that the glass flakes originated from the interior of the vial (10).
SEM of the defects on the inner surface of a representative lamellae-containing vial: patches with dark color depict surfaces defects.
ICP-MS Analysis
Solution samples in all arms, A through H, in the freeze-thaw study were analyzed by ICP-MS along with control vials of the antibody product and the placebo. A summary of the ICP-MS results can be found in Table III. All antibody vials showed comparable levels of glass leachables (Si, B, Al), regardless of freeze-thaw history. The arms with lamellae occurrence (Arms C, D, E, F) showed no difference compared to arms without lamellae formation (Arms A and B). The glass leachable levels were also consistent among all placebo vials. The source of the Na was mainly from the antibody product formulation and placebo formulation, not from the vial. The buffer used to prepare the antibody product and placebo was made from two separate manufacturing lots. The glass leachable levels were slightly different between the antibody product and the placebo samples — for example, higher Si and Al levels, and a lower B level, were present in antibody vials. This could be due to slight surface interaction differences where protein was present, due to the different stopper lots used, and/or due to the minor differences in the Na concentration. Notably, there is no correlation between high lamellae occurrence (e.g., Arms D and E) and elevated glass leachables (Si, B, Al). In addition, the glass leachable levels observed in Table III were also consistent with the historical normal ranges observed from other similar protein formulations or placebos in glass vials.
ICP-MS Results for Elements Related to Glass Leachables Measured from the Freeze-Thawed Samples (Antibody Product and Placebo) and Control Vials without Freeze-Thaw
The ICP-MS results above, which did not show a correlation between elevated levels of glass leachables and lamellae occurrence, provide further evidence that the glass lamellae formed in the frozen vials are not caused by glass dissolution from the product contact surface or glass degradation by corrosive attack (10). This conclusion is also supported by literature. For example, Iacocca et al. reported that an increase in silica concentration in the solution is an indicator of delamination resulting from chemical erosion by higher pH or terminal sterilization (14, 17). The level of Si in solution in the Iacocca study was up to 60 μg/mL (i.e., 60,000 ppb), and was over 5 times higher than what we have seen (Table III).
Additional Discussion
When considered in light of the prior work on vial breakage, the results reported here raise a fundamental question as to why vials would crack versus delaminate based on the same mechanism. The explanation is the fill volume dependence. With a given protein formulation, higher fill volume increases the vial breakage because a larger surface area is in contact with the product solution, which consequently increases the potential energy released during the plugging-off event (21). Based on the vial breakage findings, we only filled 3.5 mL in 10 cc vials with this relatively low protein concentration antibody product at 30 mg/mL. Hence no vial breakage occurred in this study. However, the unequal coefficients of expansion of the glass versus frozen protein solution still generate a lower-level stress that is sufficient to peel off small glass particles in the vials tested.
In addition to the factors evaluated in this study, container quality and processing/handling history of vials are also important. For example, high alkalinity is known to weaken glass surface and to increase potential of delamination in product vials (1, 17). Vial surface can also become susceptible to delamination as a result of the vial forming and preparation processes (14). Although this study did not evaluate these factors that affect glass vial quality, in principle, when the glass surface is weakened prior to the fill, these vials likely become more susceptible to thermally-induced mechanical stress and hence would be more prone to generate lamellae during the freeze-thaw. Therefore, it is advisable to evaluate potential glass vial quality related mitigation approaches such as selecting vials with lower alkalinity or with a more durable surface (e.g., surface coating).
In addition, the fill volume–to–vial volume ratio likely affects the probability of lamellae formation. Considering that higher vial breakage rates happened with a higher fill volume of the same formulation in any given size vial (21, 27), it would be reasonable to expect that higher fill volume would likely increase the incidence of lamellae formation during −70 °C freeze-thaws when the other parameters (formulation, vial, handling, etc.) are fixed. Vial size also plays a role, where larger size vials exhibit lower mechanical durability against vial breakage when the other parameters (fill volume–to–vial volume ratio, type of glass vial, formulation, handling, etc.) are fixed (21, 27). More heat is necessary to form the bottom of a bigger vial, so larger vials have higher probability of vial bottom containing weak spots and pre-existing defects caused by the high temperatures employed in the vial-forming process. Therefore, it is expected that larger vials would have an increased incidence of lamellae formation during −70 °C freeze-thaws compared to smaller vials with the same formulation and fill volume–to–vial volume ratio.
As an alternative to glass, it is advisable to investigate other containers possessing better elasticity, such as plastic resin vials to accommodate the thermal contraction of frozen formulations. Additionally, certain surface modifications of glass vials might change the interactions between the glass surface and protein formulation.
Conclusions
The observations of lamellae occurrence in the freeze-thaw study, the characterization of lamellae particles and vial inner surface, along with the ICP-MS testing results support the hypothesis that the flake-like glass lamellae particles formed as a result of the same mechanism as the vial breakage: the thermally-induced mechanical stress due to contraction of frozen formulations below −30 °C leading to compression on the vial surface and the abrupt separation of the glass vial inner surface from the frozen protein formulation. The glass lamellae in the frozen vials were not caused by chemical delamination where glass is eroded gradually into the solution over long-term liquid storage.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to acknowledge all certified inspectors from Amgen Clinical Manufacturing and Quality Control led by Jeff Stephens for performing the visual testing, Rick Hurtado at the Amgen warehouse for helping with the logistics of material transfers, Genevieve Tanguay in Amgen Clinical Quality Control for assisting with the lamellae filtration, Merleen Gholdston in Amgen Product & Process Development for testing protein concentration gradient in vials, Rowena Cruz and Chance Powel in Amgen Quality Assurance, and Wenchang Ji in Amgen Drug Product Engineering for operational support of this study.
- © PDA, Inc. 2013