Abstract
Part 1 of this three-part research series detailed the development and validation of a high-voltage leak detection test (HVLD, also known as an electrical conductivity and capacitance test) for verifying the container–closure integrity of a small-volume laminate plastic bag containing an aqueous solution formulation of the rapid-acting insulin analogue, insulin aspart (NovoRapid®/NovoLog®) by Novo Nordisk A/S, Bagsværd, Denmark. Leak detection capability was verified using positive controls each with a single laser-drilled hole in the bag film face. In this Part 2, HVLD leak detection capability was further explored in four separate studies. Study 1 investigated the ability of HVLD to detect weaknesses and/or gaps in the bag heat seal. Study 2 checked the HVLD detection of bag holes in packages stored 4 days at ambient conditions followed by 17 days at refrigeration. Study 3 examined HVLD test results for packages tested when cold. Study 4 compared HVLD test results as a function of bag plastic film lots. The final Part 3 of this series will report the impact of HVLD exposure on product visual appearance and chemical stability.
LAY ABSTRACT: In Part 1 of this three-part series, a leak test method based on electrical conductivity and capacitance, also called high-voltage leak detection (HVLD), was used to find leaks in small plastic bags filled with a solution for injection of the rapid-acting insulin analogue, insulin aspart (NovoRapid®/NovoLog®) by Novo Nordisk A/S, Bagsværd, Denmark. In this Part 2, HVLD leak detection capability was further explored in four separate studies. Study 1 investigated the ability of HVLD to detect bag heat seal leaks. Study 2 checked HVLD's ability to detect bag holes after a total of 21 days at ambient plus refrigerated temperatures. Study 3 looked to see if HVLD results changed for packages tested when still cold. Study 4 compared HVLD results for multiple bag plastic film lots. The final Part 3 of this series will report any evidence of drug component degradation caused by HVLD exposure.
- Container-closure
- Container-closure integrity
- Defects
- Electrical conductivity and capacitance leak detection
- Form-fill-seal packages
- High-voltage leak detection
- HVLD
- Leak
- Leakage
- Leak detection
- Leak test method
- Package
- Package defects
- Package integrity
- Package integrity method
- Plastic laminate bag
- Protein product
- Stability
- Insulin.
Introduction
Part 1 of this research series documented the development and validation of six high-voltage leak detection (HVLD) method options for leak testing small-volume plastic bags filled with the rapid acting insulin analogue, insulin aspart (NovoRapid®/NovoLog®) by Novo Nordisk A/S, Bagsværd, Denmark. Part 2 further explored the leak detection capability of HVLD in four studies:
Study 1. Detection of heat seal defects: Part 1 focused on the detection of laser-drilled hole defects in the bag film face. In Part 2 Study 1, the ability of HVLD to find weaknesses or gaps in the bag heat seal was investigated.
Study 2. Detection of hole defects as a function of product-package storage: Part 1 employed product–filled packages filled and tested at ambient temperature conditions. Validation studies utilized positive controls (with-leak bags) tested the day of package fill, using product at ambient temperature. However, optimal active drug physico-chemical stability necessitates long-term product–package storage at 5 ± 3 °C. Therefore, Study 2 explored the ability of HVLD to find package holes using product-filled packages stored for 4 days at ambient temperatures followed by 17 days at refrigeration (5 ± 3 °C).
Study 3. HVLD of cold product–packages: As noted above, product–packages are typically stored at 5 ± 3 °C. In all earlier reported work, HVLD tests were performed on ambient temperature product-filled bags. Study 3 examined HVLD results for negative control product–packages leak tested when still cold, immediately following refrigerated storage.
Study 4. HVLD as a function of package plastic laminate lot: Finally, Part 2 tested no-defect bags representing multiple plastic laminate lots to see the impact of this variable on HVLD test results.
All Part 2 HVLD studies were performed at Whitehouse Laboratories (WAL, Whitehouse, NJ) under the direction of consultant Dana Guazzo of RxPax, LLC (Bridgewater, NJ). The HVLD instrument was a Nikka Densok USA, Inc. HDT-1 pinhole inspector (Lakewood, CO) equipped with a data acquisition system and other miscellaneous equipment parts designed and created by TellTech Service Corporation (Mahopac, NY). Packaging Technologies & Inspection, LLC (Tuckahoe, NY) provided collaborative support for the instrument and equipment suppliers, the testing lab, and Novo Nordisk A/S. Package hole creation and hole size certification was performed by Lenox Laser (Glen Arm, MD). Novo Nordisk A/S of Bagsværd, Denmark fully subsidized all work.
Materials and Methods
1. Product–Package Test Units
The same product–package types (also called product-filled bags or units) described and illustrated in Part 1 were used in Part 2. As in Part 1, Novo Nordisk–made units were filled with the product and were sealed by an automatic form-fill-seal process. WAL-made units were manually filled and sealed at WAL by the process detailed and illustrated in Part 1, with metal clips applied for those requiring this addition, as per study protocol. With-clip bags had a metal clip crimped onto the bag septum-end; no-clip bags had no clip attached.
For all Part 2 studies, negative controls were included, which are product–packages with no intentional defect (i.e., leak). Negative controls in Studies 1 and 2 were made from one laminate film lot. Studies 3 and 4 included negative control units representing three different laminate film lots.
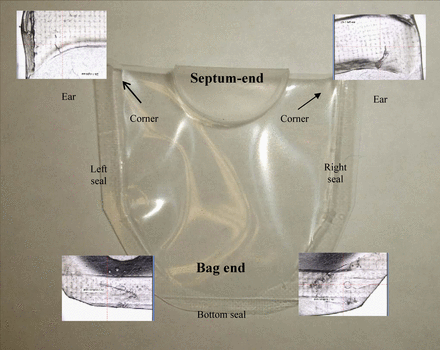
Positive controls used in Part 2 included two types: (1) heat seal defect units (for Study 1), and (2) laser-drilled hole defect units (for Study 2). Study 1 heat seal defects included pilot plant manufactured bags with seal anomalies at the bag ears, unintentionally introduced in the seal by Novo Nordisk A/S, and culled out at WAL by visual inspection. In addition, weakly bonded seals were intentionally formed along the bag bottom edge by setting the WAL heat sealer to dwell times less than optimal. No Study 1 seal defects were sized, but were generally categorized as largely-leaking defects. In Figure 1 a bag is shown with magnified images of representative heat seal defect types included.
Empty package with bag regions noted and magnified images of defect types.
Study 2 positive controls each had a single laser-drilled hole located in the bag corner to the right of the septum. All Study 2 positive controls were stored at ambient temperatures for 4 days followed by refrigeration (5 ± 3 °C) for 17 days prior to HVLD testing. Additional details regarding each study's positive and negative controls are noted in Results and Discussion.
Test System and Test Methodology
Part 1 provides a full description of the HDT-1 Pinhole Inspector manufactured by Nikka Densok USA, Inc. used for all HVLD tests. Part 1 also delineates test instrument set-up, operation, test sample positioning, and test method variables.
Validated HVLD Test Methods
Six different HVLD test methods were developed and validated as reported in Part 1 of this series. The final validated methods are outlined in Table I.
HVLD Test Methods
As discussed in Part 1, six methods were developed and validated, specific to the following package/testing scenarios: (1) the presence or absence of the metal clip on the bag, (2) for with-clip bags, the bag orientation on the test platform (clip up or down), and (3) the direction through which the bag is conveyed through the testing zone (septum end first or bag end first). For each method, specific acceptance criteria apply. HVLD voltage readings that fall within the Reference Minimum (Ref Min) and Reference Maximum (Ref Max) ranges are indicative of passing results (no leak detected), while voltages equal to or greater than Ref Max are indicative of failing results (leak detected). HVLD test results equal to or less than Ref Min serve as a warning indicator of possible system operation drift or test sample misalignment. The method(s) used in each study are noted in Results and Discussion. (Note: Routine manufacturing will employ only one test to check each bag face for leaks.)
Results and Discussion
Study 1. Detection of Heat Seal Defects
Bag Ear Defects
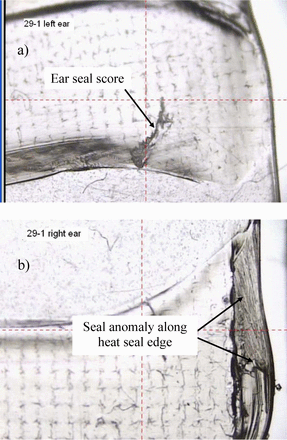
Visual abnormalities in bag ear heat seals were identified at WAL by microscopic examination of prefilled bags. Examples of bag ear anomalies are shown in Figures 2 and 3. Ear-defect bag samples were filled with product and manually heat-sealed along the opposite bottom end (refer to Part 1 for filling/assembly process). Metal clips were applied to packages as specified by the HVLD method. Bags with ear anomalies were evaluated by HVLD, along with a negative control bag used intermittently to verify HVLD baseline performance.
Bag 29-1 ear seal defects, magnified images. a) Ear score, b) Ear seal anomaly.
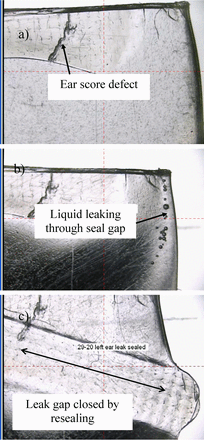
Bag 29-20 ear seal defects, magnified images. a) Score defect, b) Leak path allowing liquid flow upon bag squeezing, c) Leak path heat sealed to block liquid flow.
HVLD test results of bags with ear defects are presented in Table II. Data sets 1 and 2 show that units with bag ear scores and other anomalies that did not exude visible liquid leakage upon bag squeezing passed HVLD tests, suggesting such defects are cosmetic and pose no risk to package integrity. HVLD failure only occurred when seal gaps permitting visible liquid leakage were present (sets 3 to 5). In these cases, heat sealing the leaking bag ear resulted in passing HVLD tests (sets 4 and 5).
HVLD of Bag Ear Heat Seal Defects
Both bags in set 5 had visible leakage that the HVLD test failed to find when performed by Method 2 (no clip, bag first); when conveyed through the test zone in the opposite direction, both leaks were found (Method 1: no clip, septum-first). Applying metal clips to the bags improved leak detection regardless of test direction and clip orientation (Methods 3 through 6). These limited results imply better HVLD detection of bag ear leaks if tests are performed septum first; metal clip presence may also improve bag ear leak detection.
Bag Bottom Seal Defects
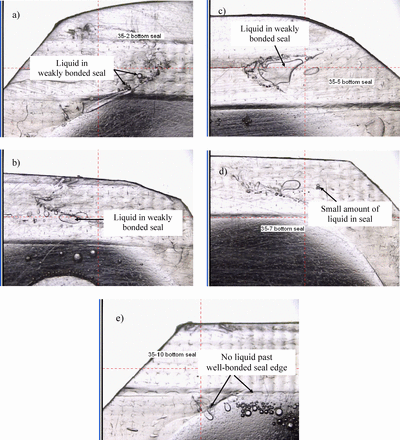
Bags without any apparent defect were filled and sealed along the bottom edge at WAL using heat seal dwell time set points bracketing the optimal set point of 6.5 (seal examples are shown in Figure 4). Finished units were tested by HVLD by Methods 1 and 2, along with a negative control optimally sealed to verify HVLD baseline performance.
Bag bottom seals as a function of heat seal dwell time, magnified images. a) Bag 35-2 (setting 3.5), b) Bag 35-3 (setting 4.0), c) Bag 35-5 (setting 4.5), d) Bag 35-7 (setting 5.0), e) Bag 35-10 (setting 8.0).
Table III results indicate HVLD was able to detect leak paths that allowed visible liquid passage, sealed at the worst-case set point 3.5. Both bags sealed at set point 4.0 were identified as leaking, despite the absence of visible leakage. One of the two bags sealed at setting 4.5 visibly leaked; both bags were identified as leaking 1 of 2 times by each Method 1 and 2. Bags prepared at settings 5.0 and above passed all HVLD tests. In conclusion, HVLD is able to detect leaks located in the bag seals. The likelihood of seal leak detection increases as a function of heat seal deterioration and the presence of liquid in the leak path.
HVLD of Poorly Sealed Bags
Study 2. Detection of Hole Defects as a Function of Product–Package Storage
Physico-chemical stability of the proteinaceous active drug component of the bagged solution formulation is best assured by long-term storage of the product–package at refrigeration with limited exposure to higher, ambient temperature conditions. Therefore, Study 2 explored the ability of HVLD to identify leaks in product-filled packages after extended ambient plus cold temperature storage. This question is especially relevant because protein-based compounds tend to clog leak paths. Clogged leaks may be missed by other test methods that rely on the flow of liquid or gas through the leak path for detection (e.g., dye ingress, mass extraction, and vacuum decay).
The same positive controls used for Part 1 Method Validation Day 1 tests were used for Study 2. The laser-drilled hole sizes ranged from 2.7 to 10.5 μm in nominal diameter and represented six bag locations (left and right seal edges, left and right corners, bottom seal, and center). Following Validation Day 1 tests, these positive controls were kept in an open laboratory environment at ambient conditions for 4 days. Afterward, these bags were stored at 5 ± 3 °C for an additional 17 days for a total of 3 weeks. On the final day, bags were allowed to come to ambient temperature, and were tested by HVLD within a few hours of cold storage removal, along with negative control bags. Testing order duplicated that of Method Validation Day 1.
Test results are found in Table IV, and are highlighted as follows.
HVLD Reliability for Product–Packages Stored 4 Days (Ambient) Plus 17 Days (2–8 °C)
Methods (1, 2, 5, and 6) accurately identified all negative and positive control test samples. (Method 2 negative control bag 33-23 “failed,” but a leak discovered at the bag's seal edge confirmed the accuracy of this result.)
Method 3 gave one false-positive result out of 12 tests performed (Bag 34-6). An occasional false positive is not surprising given that the Ref Max acceptance criterion was set 3 standard deviation units above the negative control mean (refer to Part 1 for details). This limit was intentionally chosen, as the impact of falsely rejecting a good package was considered inconsequential compared to that of missing a leaking one.
Method 4 tests yielded one false-negative result in 12 tests performed (Bag 38), although a retest produced a fail result. This same holed sample also avoided detection during Day 1 Validation tests, likely due to the hole's location on the partially collapsed bag surface. As confirmed in Part 1, a leak in a bag partially or fully collapsed due to low fill or product leakage might evade detection if HVLD electrode brushes fail to contact the defective area during test. Screening packages for improper fill can eliminate this risk of incorrectly passing largely leaking packages.
To conclude, storage of product-filled bags for up to 21 days, including 4 days at ambient temperatures and 17 days at refrigeration, had no measurable impact on HVLD reliability. With the exception of a single false-positive test result, HVLD tests performed on product-filled bags the day of fill-and-seal mirrored those generated after a 21-day bag storage period. This finding is significant given the proteinaceous nature of the active component, known from past experience by Novo Nordisk A/S that this product formulation tends to clogs leak paths, making leak detection by other test methods such as vacuum decay unreliable.
Study 3. HVLD of Cold Product–Packages
All prior study HVLD tests were performed on ambient temperature product-filled bags. Study 3 explored the consistency of HVLD readings for no-defect product-filled bags if tested when still cold from refrigerated storage.
Test units were all product-filled negative control bags prepared from three laminate film lots (designated A, B, and C). During 5 ± 3 °C storage, bags were spaced apart laying face-down on metal trays. The applicable metal tray with bags was removed from refrigerated storage immediately prior to HVLD test start. Each bag was manually transferred from the metal tray to the HVLD test carriage, touching only the outer bag edges. Bags were either tested in “whole sets” (meaning all test bags to be tested by a given method were removed from cold storage simultaneously, then sequentially tested), or they were tested in multiple “subsets” (meaning a smaller group of bags was removed from refrigeration at the same time, then each bag was sequentially tested). The same bags used for whole set tests were also used for subset tests. HVLD tests started within 1 min after bag set storage removal. All data are time-stamped with the first bag test as time zero. Two days of replicate tests were performed. All six HVLD methods were included, although only one bag lot was used per method. Bag lots were matched with methods to ensure the two no-clip methods were evaluated by two different bag lots, and the three with-clip methods were evaluated by at least two different bag lots.
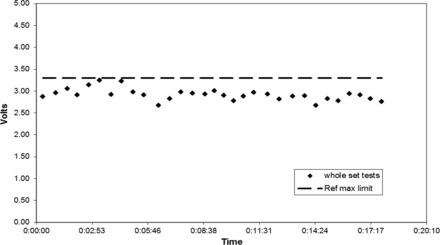
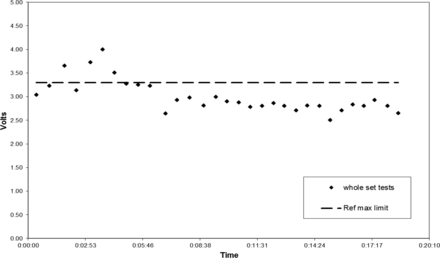
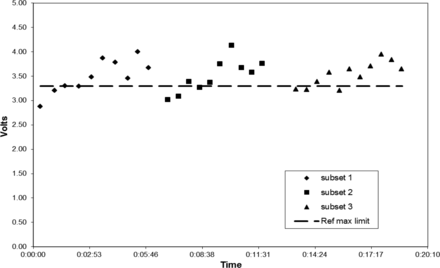
All six HVLD test methods yielded similar data. For brevity, only Bag lot C Method 6 test results are graphically illustrated (Figures 5 to 7). Sequential HVLD voltage readings of the bags tested as one set started low, slowly climbed, and then declined (Figures 5 and 6). This peaking effect was confirmed to correlate to time post-refrigerated storage. When all bags were removed from the refrigerator simultaneously (i.e., as a whole set), one peak was observed (Figures 5 and 6). When these same bags were placed back in the refrigerator and were removed in three smaller subsets, three peaks were observed (Figure 7). In all cases, maximum voltage was observed for bag tests time-stamped within about 3 min post-refrigerated storage removal. Often, highest readings exceeded the Ref Max limit. Interestingly, the rise in voltage readings corresponded to condensation observed on cold bag surfaces upon exposure to room temperature and humidity conditions. Bag surface condensation disappeared about 5 to 6 min post-refrigeration, as voltage readings returned to baseline values, even though bags still felt cool. Peak voltage readings varied from day to day as Figures 5 and 6 illustrate. This variation in maximum peak voltage reading is likely a function of test area relative humidity; however, no exact correlation was attempted. In summary, this work found that bag surface condensation will elevate HVLD voltage readings temporarily, risking false-positive test results. To avoid this, refrigerated bags should be allowed to warm at temperature and humidity conditions that preclude bag surface moisture prior to HVLD tests.
Bag lot C (whole set) tested post refrigeration by HVLD Method 6, Day 1.
Bag lot C (whole set) tested post refrigeration by HVLD Method 6, Day 2.
Bag lot C (3 subsets) tested post refrigeration by HVLD Method 6, Day 2.
Study 4. HVLD as a Function of Package Plastic Laminate Lot
Study 4 explored the effect of laminate film manufacturing lot on HVLD test results. About 33 WAL-made negative controls were prepared for each of three bag film lots, designated lots A, B, and C. All three lots were sourced from a single converter, but lot B ingredients came from a different raw material supplier. All negative controls were HVLD-tested by all six methods on 3 replicate test days. Bags were stored between tests at ambient conditions.
Results are summarized in Table V. Lots A and C performed similarly with no more than 2 units exceeding the Ref Max limit by any given method over 3 test days. Each lot A and C also had one unit to fall below the Ref Min warning limit when tested by Method 1. In contrast, lot B yielded markedly more failing results. This was especially apparent for test Methods 1, 3, 4, and 6, in which false-positive rates ranged from 5% (Method 3) to 15% (Method 1). Voltage reading coefficients of variation were similar for the three lots; therefore, the rise in lot B false positives was not due to greater data variability, but to an upward shift in the HVLD baseline for lot B. The unique characteristic(s) of lot B affecting HVLD readings could not be pinpointed. However, to ensure consistency in HVLD results, steps would be required to confirm bag HVLD performance prior to approving a laminate film supplier.
HVLD as a Function of Package Plastic Laminate Lot
Conclusions
This three-part research series explored the method development, validation, and application of HVLD for container–closure integrity analysis of a uniquely designed, small-volume plastic bag intended as a primary container for an injectable solution of a proteinaceous insulin analog active ingredient. Part 1 documented the HVLD method development process, and presented data validating the ability of this technology to find leaks caused by laser-drilled hole defects in the bag film.
The current Part 2 further challenged HVLD as a leak detection tool for this package system. The laser-drilled hole defects used in Part 1 were similar in shape and were each sized for nominal diameter. Such well-controlled defects of defined-size enabled meaningful data interpretation during method development and validation work. However, most naturally occurring defects are not well-defined holes in the plastic wall. Therefore, Part 2 (Study 1) explored the ability of the validation HVLD methods to find various types of package heat seal defects similar to what might occur during routine production. Results found that larger leaks due to bag seal anomalies or weakly bonded heat seals located at either end of the bag could be identified by HVLD. Detection is more likely when liquid is present in seal gaps or in weakly bonded seals. For bag ear seals, leak detection may be improved with metal clip presence.
All earlier HVLD tests described in Part 1 of this series were performed on ambient temperature product–packages, with positive controls filled the same day. However, the NovoRapid®/NovoLog®product stability requires storage at temperatures 5 ± 3 °C. Company manufacturing practices included the option of storing product-filled packages for a few days at room temperature conditions, followed by storage at 5 ± 3 °C for up to 2 weeks prior to integrity testing and final product release. Therefore, Study 2 was designed to support these practices. In Study 2, holed positive controls were tested by HVLD after being stored for 4 days at ambient temperatures followed by 17 days at refrigeration (5 ± 3 °C). In this study, HVLD tests were performed after the refrigerated units warmed to ambient temperature. Findings showed no difference in leak detection reliability between these units versus those tested on the day of package fill. This finding is especially noteworthy, as Novo Nordisk A/S research found the proteinaceous formulation clogged leak paths preventing their detection by other commonly used methods such as vacuum decay.
All previous HVLD tests were performed on packages maintained at ambient temperature. Study 3 explored the impact of testing product–packages immediately after refrigeration, when still cold. Only negative control units without defect were included. Findings showed voltage readings were elevated when cold package surfaces were visibly moist from condensation; cold surface temperature alone had no influence. Thus to avoid falsely rejecting packages, data suggest HVLD should be performed at conditions preventing package surface condensation.
It is important to note that Studies 2 and 3 reference specific manufacturing practices of Novo Nordisk A/S. These data are provided to help the reader understand HVLD method capabilities. However, such studies may not be necessary for on-line HVLD method development for other products. On the other hand, if HVLD methods are intended for product stability testing, studies of refrigerated samples and samples post long-term storage may be required. Needs to perform such special studies should be evaluated based on how the HLVD method will be applied and what samples will be tested.
Study 4 explored the effect of plastic bag film lot on HVLD voltage readings. Using negative controls created from three different laminate lots, a shift in baseline results by all six HVLD methods was observed for one of the three lots. Although the difference among plastics causing this shift was not determined, it was clear that material source may influence HVLD baseline readings.
Finally, this research series describes Part 2 as having occurred subsequent to Part 1 HVLD method development and validation. Research findings were organized in this manner for better reader understanding. But in fact, some of the variables of Part 2 were explored in conjunction with HVLD method development, and these findings played an important part in determining the appropriate application of HVLD in assuring product–package quality. Package integrity cannot be assured simply by a single leak test method. Leak testing is part of a package integrity assurance process that starts with product–package design and ends with product consumption. Further, leak test method development does not follow a prescriptive course identical for all product–packages and test methods. Multiple factors should be considered, including package material composition and design, product formulation, the types of defects requiring detection, and final test method application.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2013