Abstract
This Part 3 of this three-part research series reports the impact of high-voltage leak detection (HVLD) exposure on the physico-chemical stability of the packaged product. The product, intended for human administration by injection, is an aqueous solution formulation of the rapid acting insulin analogue, insulin aspart (NovoRapid®/NovoLog®) by Novo Nordisk A/S, Bagsværd, Denmark. The package is a small-volume form-fill-seal plastic laminate bag. Product–packages exposed to HVLD were compared to unexposed product after storage for 9 months at recommended storage conditions of 5 ± 3 °C. No differences in active ingredient or degradation products assays were noted. No changes in any other stability indicating parameter results were observed. This report concludes this three-part series. Part 1 documented HVLD method development and validation work. Part 2 explored the impact of various package material, package temperature, and package storage conditions on HVLD test results. Detection of leaks in the bag seal area was investigated. In conclusion, HVLD is reported to be a validatable leak test method suitable for rapid, nondestructive container–closure integrity evaluation of the subject product–package.
LAY ABSTRACT: In Part 1 of this three-part series, a leak test method based on electrical conductivity and capacitance, also called high-voltage leak detection (HVLD), was proven to find hole leaks in small plastic bags filled with a solution of insulin aspart intended for human injection (NovoRapid®/NovoLog® by Novo Nordisk A/S, Bagsværd, Denmark). In Part 2, the ability of the HVLD method to find other types of package leaks was tested, and the impact of package material and product storage temperature on HVLD results was explored. This final Part 3 checked how well the packaged protein drug solution maintained its potency after HVLD exposure over 9 months of storage under long-term stability conditions. Results showed that HVLD caused no harm to the product.
- Container-closure
- Container-closure integrity
- Defects
- Electrical conductivity and capacitance leak detection
- Form-fill-seal packages
- High-voltage leak detection
- HVLD
- Leak
- Leakage
- Leak detection
- Leak test method
- Package
- Package defects
- Package integrity
- Package integrity method
- Plastic laminate bag
- Protein product
- Stability.
Introduction
Part 1 of this research series documented the development and validation of six high-voltage leak detection (HVLD) method options for leak-testing plastic bags filled with an aqueous solution of the rapid acting insulin analogue, insulin aspart (NovoRapid®/NovoLog®) by Novo Nordisk A/S, Bagsværd, Denmark. Part 1 employed positive controls with laser-drilled holes in the bag film.
Part 2 found that HVLD is able to identify weaknesses or gaps in the bag seals as well. Part 2 also reported that package holes could be found even after allowing 21 days of ambient plus refrigerated storage for protein to clog and block leak paths. As discussed in Part 1 of this series, earlier research performed by Novo Nordisk A/S (not reported) found that product formulation readily clogged leak paths making them undetectable by vacuum decay, which lead to the investigation of HVLD as a container–closure integrity test. No tests were performed to prove that the leaks in the samples tested by HVLD post ambient and refrigerated storage were indeed clogged.
Testing packages for leaks while still cold from refrigerated storage could temporarily elevate HVLD results due to condensation on package surfaces; this effect can be avoided by testing bags dry and at ambient temperatures. Subtle material differences in plastic laminate film lots linked to alternative material sources were found to cause an upward shift in baseline HVLD voltage readings for no-defect packages. This highlighted the importance of testing multiple package lots from multiple sources when establishing HVLD test parameters, and to check HVLD test performance prior to package supplier approval.
This final Part 3 investigated the impact of HVLD exposure on the visual appearance and chemical stability of the packaged product. The product used is the rapid-acting insulin analogue, insulin aspart (NovoRapid®/NovoLog®) by Novo Nordisk A/S, Bagsværd, Denmark. In general, electrical conductivity and capacitance leak tests are considered nondestructive to product contained in HVLD-exposed packages. Published research has shown that in rare instances HVLD exposure has triggered headspace ozone formation in small volume vials causing product oxidation (1). Therefore, prudence dictated that the influence of HVLD exposure on the visual appearance and chemical stability of insulin products be verified prior to adopting HVLD as a nondestructive container closure integrity test.
(Note: In Part 1 of this series, method validation results indicated a small risk of falsely rejecting non-leaking packages exists. One way suggested to address a questionable fail result is to retest the package after instrument performance has been confirmed using a small set of negative controls. Support for such a product retest program would require long-term stability verification of product packages exposed to HVLD at least as many times as the retest program would allow. A retesting program was not adopted by Novo Nordisk A/S; therefore, the stability studies described in Part 3 study did not include samples exposed to HLVD more than twice.)
Part 3 research, including HVLD tests, product stability storage, and visual appearance, was performed by Novo Nordisk A/S of Denmark. The HVLD instrument is a Nikka Densok HDT-1 pinhole inspector (Lakewood, CO) with data acquisition system by TellTech Services Corporation (Mahopac, NY). Dana Guazzo of RxPax, LLC (Bridgewater, NJ) provided consult support and assisted in publication preparation. All work was fully subsidized by Novo Nordisk A/S.
Materials and Methods
Product–Package
The same product-package types (also called product-filled bags or units) described and illustrated in Part 1 were used in Parts 2 and 3. For Part 3, all units were created by Novo Nordisk A/S, being filled and sealed by an automatic form-fill-seal process. Package fill volume (3.56 mL ± 4%) and headspace volume (virtually 0 mL) were typical of product manufactured on larger-scale production lines. Six different drug product lots were filled into packages representing two plastic laminate lots (one lot for batches 1–3 and another for batches 4–6). Drug product lots 4–6 were exposed to HVLD while drug product lots 1–3 were unexposed to HVLD. The six product lots were identical in formulation, bulk solution manufacture, and package assembly processes.
The classic approach for such a study would be to divide each product–package lot into an HVLD-exposed group and an HVLD-unexposed group. However, the first three lots were produced before HVLD was available, while the remaining lots were prepared after HVLD introduction. Given the profound knowledge of insulin stability in cartridge package systems collected over 20 years, the primary goal of the current work was to compare stability of HLVD-exposed and HLVD-unexposed lots to the well-established stability profile of insulin in a cartridge package.
The product itself is the rapid-acting insulin analogue, insulin aspart (NovoRapid®/NovoLog®) by Novo Nordisk A/S. NovoRapid®/NovoLog® is a sterile, aqueous, clear, and colourless solution that contains insulin aspart 100 units/mL, glycerin, phenol, metacresol, zinc, disodium hydrogen phosphate dihydrate, sodium chloride, and water for injection.
HVLD Test System and Test Methodology
Part 1 provides a full description of the HDT-1 Pinhole Inspector manufactured by Nikka Densok, Inc. used for Parts 1 and 2 HVLD tests. For Part 3, an identical HVLD unit was used by Novo Nordisk A/S to leak test product–packages. Each unit from the drug product lots intended for HVLD exposure was checked twice. Methods 5 and 6 were used for testing the bags (Test Methods are described in Part 1, Table I).
Slope Estimates for the Insulin Aspart Assay Linear Regression Lines and the 95% Confidence Intervals
Product Stability Storage and Analysis
Product–packages (both HVLD-exposed and non-exposed) were stored for 9 months at long-term stability conditions (5 ± 3 °C). Initially and over time (0, 3, 6, and 9 months), product was removed from storage and evaluated according to the following tests. One product–package from each of the six drug product batches was analyzed for each time point. As the product is approved for commercial market (marketed as NovoRapid®/NovoLog® 100 units of insulin aspart per mL in vials and cartridges), routine quality control methods approved by European health authorities and the U.S. Food and Drug Administration were performed:
Assay of insulin aspart
Insulin aspart–related impurities
High molecular weight proteins (dimers and polymers of insulin aspart)
Desamido insulin aspart
B28IsoAsp insulin aspart
Bacterial endotoxins
Freezing point depression
Preservative content (phenol and metacresol)
Zinc content
Particulates
Macroscopic appearance
pH
Sterility
Results and Disussion
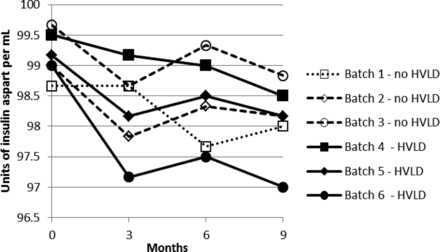
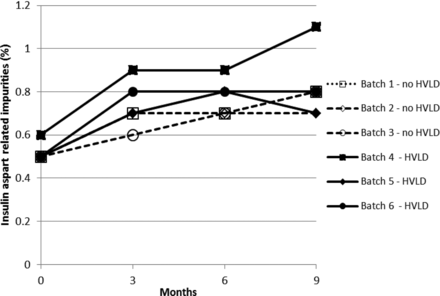
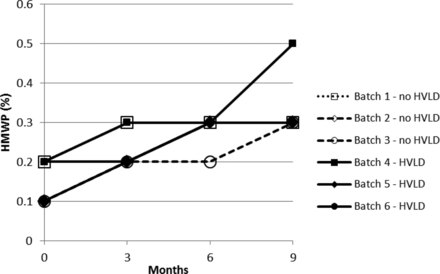
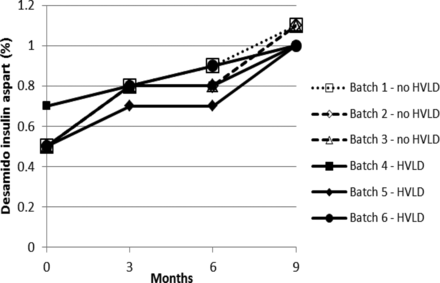
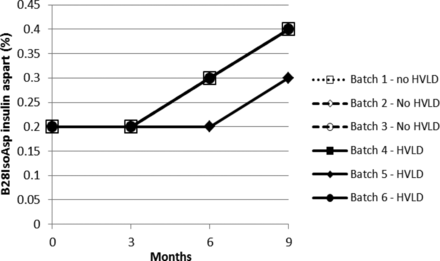
Stability test results obtained for drug product solution exposed and not exposed to HVLD and stored for 9 months at 5 ± 3 °C are graphically illustrated in Figures 1 through 5. Figures 1 and 2 include insulin aspart assay and related impurities. Figures 3 through 5 detail results for high molecular weight proteins (dimers and polymers of insulin aspart), Desamido insulin aspart, and B28IsoAsp insulin aspart, respectively.
Insulin aspart assay.
Insulin aspart related impurities (refer to Table II).
High molecular weight proteins (HMWP) (refer to Table III).
Desamido insulin aspart (refer to Table IV).
B28IsoAsp insulin aspart (refer to Table V).
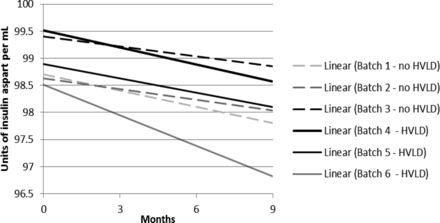
For assay of insulin aspart a regression plot (or linear regression) was made for each of the six individual production lots (Figure 6). A 95% confidence interval for the slope estimate was estimated. The 95% confidence interval (lower and upper) for the slope estimate is given in Table I. There is no statistically significant difference between the assay slopes in the two groups (lots treated with HVLD and lots not treated with HVLD) (P-value = 0.33). In other words, HVLD treatment demonstrated no effect on assay of insulin aspart (admittedly, the dataset is small). The data illustrated in Figures 2 through 5 were further evaluated by linear regression analysis and the 95% confidence intervals for each slope were estimated as documented in Tables II through V, respectively. No difference was noted among HVLD-treated lots and lots not treated.
Linear regression plot of the six drug product lots (refer to Table I).
Insulin Aspart Related Impurities 95% Confidence Interval for Each Slope Estimate
Insulin Aspart Related Impurities (Result Unit: %)
High Molecular Weight Proteins 95% Confidence Interval for Each Slope Estimate
High Molecular Weight Proteins (Result Unit: %)
Desamido Insulin Aspart 95% Confidence Interval for Each Slope Estimate
Desamido Insulin Aspart (Result Unit: %)
B28IsoAsp Insulin Aspart 95% Confidence Interval for Each Slope Estimate
B28IsoAsp Insulin Aspart (Result Unit: %)
For the remaining analytical parameters, all results are similar within the analytical variation. In addition, all other parameters tested remained within product specification limits throughout the 9 month study. These include bacterial endotoxin, freezing point depression, macroscopic appearance, preservative content, particulates, zinc content, and pH.
Conclusions
This study concluded a three-part research series designed to explore the use of HVLD as a nondestructive container–closure integrity test for small-volume plastic laminate bags made to contain an insulin solution for injection by Novo Nordisk A/S of Bagsværd, Denmark. In Part 1, the development and validation of HVLD methods optimized for detecting leaks in the bag film was described. In Part 2, various factors were explored for their potential impact on HVLD tests. These included package lot, package temperature, and package storage conditions. In addition, the ability of HVLD to find leaks in the bag seal area was examined.
In Part 3, three drug product lot packages leak tested by HVLD were placed on stability and compared to three drug product lots not HVLD-tested over a 9 month storage period at 5 ± 3 °C. Each HVLD-exposed product-filled package was HLVD-tested twice in order to check for leaks on both bag face sides. Results found no statistically significant differences in active, impurities, or degradation products assays. No statistically significant differences in all other stability indicating parameter results were reported. All data remained within commercial product specification limits. These data indicate that HVLD is a validatable leak test method suitable for rapid, nondestructive container–closure integrity evaluation of a small-volume plastic laminate form-fill-seal bag filled with the rapid-acting insulin analogue, insulin aspart (NovoRapid®/NovoLog®) by Novo Nordisk A/S.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2013
Reference
- 1.↵