Abstract
Leachable tungsten is associated with protein aggregation and precipitation in glass prefilled syringes, and this may trigger immunogenicity concerns. Determining the level of leachable tungsten from glass prefilled syringes is critical for assuring quality of certain biopharmaceutical drug products. An inductively coupled plasma mass spectrometry (ICP/MS) quantification method was developed to determine elemental tungsten in syringe extracts. The syringe was extracted using 0.5% ammonium hydroxide (pH 11), heat (75 °C), and sonication. The resulting extraction solution was diluted 10 fold prior to ICP/MS analysis. Syringes from three syringe lots containing known low (average 28.0 ng), medium (average 189.4 ng), and high (average 631.9 ng) levels of tungsten were extracted three times each. All syringes with total tungsten greater than 14 ng had extraction efficiency greater than 90% with the first two extractions combined. The calibration curve range was 0.1–200 μg/L tungsten with iridium as the internal standard, and the correlation coefficient was ≥1.0000. The limit of detection at 0.05 μg/L tungsten and limit of quantification at 0.1 μg/L tungsten were determined as having a signal-to-noise ratio greater than 40 and 80 times compared with the blank, respectively. The ICP/MS method was selective for tungsten and iridium in the presence of other metals. Accuracies of spiked tungsten, at three different levels, in syringe extracts were >99% with precision relative standard deviation (RSD) (n = 5) of ≤1%. The matrix effect of the syringe extract media and carryover of tungsten and internal standard were negligible. Onboard stability of the syringe extracts over three days had a tungsten concentration RSD (n = 3) of ≤1%. Syringe extractions performed with 0.45–0.55% ammonium hydroxide had spike recoveries ≥99% and demonstrated extraction solution robustness. Quantified residual tungsten in syringes extract by ammonium hydroxide and analyzed by ICP/MS was acceptable based on extraction efficiency and method performance.
LAY ABSTRACT: Elemental tungsten is a known leachable from glass prefilled syringe used as a ready-to-inject drug device in the pharmaceutical industry. Tungsten is a residual artifact from the manufacturing process of the syringe. The leachable tungsten level is of a concern, as it can affect the quality of the filled drug product. To understand possible leachable quantity of tungsten from the prefilled syringe, a tungsten extraction conditions and quantification method were developed. Double extraction of the syringe with 0.5% ammonium hydroxide (pH 11), heat (75 °C), and sonication was able to efficiently extract 90% of the total tungsten from syringe. An inductively coupled plasma mass spectrometry method was qualified to selectively, accurately, and precisely quantify the extracted tungsten. The developed extraction and quantification method was acceptable in determining possible leachable tungsten from prefilled syringes.
I. Introduction
Glass prefilled syringes (PFSs) are increasingly becoming a container of choice for storing and administering various therapeutic protein products to patients (1⇓–3). The PFS is susceptible to leaching from multiple syringe components (glass barrel, stainless steel hypodermic needle, rubber needle shield, and Teflon coated rubber plunger), silicone oil lubricant, from less likely sources such as residues from processing tools (4, 5), and adhesives for attaching the needle to the barrel (5, 6). Leachables associated with tungsten, silicone oil, and adhesive species are known to cause protein aggregation, particle formation, and/or react with the formulation or protein (4, 7⇓⇓⇓⇓–12).
Tungsten leaching from a PFS is known to induce protein particle formation (4). The source was traced to the tungsten pins used in the manufacturing process of the syringe barrels. Tungsten pins are used to shape the glass syringe tip and make the syringe opening, where the needle is affixed. During the process of making the syringe tip opening, tungsten pins are in contact with heated borosilicate glass at near 1200 °C. This process oxidizes the tungsten pin surface and also transfers alkali borate from the glass to the tungsten pins. Tungsten oxides sublime at >500 °C and deposits in the cooler inner surface of the syringe tip opening (4). Pure tungsten oxide residues in PFS are unlikely to leach out at concern levels due to their limited solubility. But, exposure of tungsten pins to borosilicate glass at 1200 °C may form sodium polytungstate species (4, 8), which are water-soluble and may leach under normal conditions of PFS use and subsequently cause formation of protein particles and aggregates.
The tungsten-induced protein particle formation has been studied by spiking two model proteins with tungsten pin extracts and commercially available sodium tungstate (8). Tungsten pin extracts were more effective in causing protein aggregation than commercial tungsten standard, suggesting that protein aggregation likely resulted from electrostatic interaction, interaction increased at lower pH, and aggregation is protein-dependent (8). Precipitation of a monoclonal antibody by soluble tungsten was also observed (9), and tungsten-induced precipitation should only be a concern for proteins formulated below pH 6 because tungsten polyanions are not formed at higher pH. More recently, tungsten was linked to immunogenicity of Epoetin alfa in prefilled syringes (10). It was proposed that tungsten-mediated unfolding and aggregation of Epoetin alfa as a potential root cause for increased immunogenicity and that the finding might be more broadly applicable to other classes of therapeutic proteins.
Improved syringe barrel forming and washing processes at the supplier have lowered the residual tungsten content and significantly reduced the risk of protein aggregate formation. Screening PFS lots for tungsten prior to drug filling may reduce unnecessary waste of drug product. There is currently no published analytical technique to quantify extractable tungsten from PFSs. An inductively coupled plasma mass spectrometry (ICP/MS) analytical test methodology with an efficient tungsten extraction method to determine tungsten in individual glass PFSs is described. The ICP/MS technique was selected as the analytical technique based on its established capability for specific and trace-level elemental quantification (13).
II. Materials and Methods
Materials
Commercially available, glass PFS 1 mL barrel and plungers were obtained from a syringe manufacturer. Tungsten (W) and iridium (Ir) elemental standard solutions were from Aldrich (St Louis, MO). Platinum (Pt) elemental standard was from Inorganic Ventures (Christiansburg, VA). Osmium (Os) and Rhenium (Re) elemental standards were from BDH (West Chester, PA). TraceSELECT grade ammonium hydroxide, >25% was from Fluka (St Louis, MO). Milli-Q water from a laboratory water purification system (Millipore, Billerica, MA) was used.
Tungsten Extraction
Residual tungsten deposits not removed by the supplier's wash processes are likely to be located in the syringe funnel region. Various parameters in extracting tungsten from the syringes were considered including extraction solution (acid, water, base), assembly (with/without needle shield), technique (incubation, sonication), temperature (room temperature to 75 °C), and time (zero hour to overnight incubation/sonication). Tungsten extraction efficiency of each examined set of parameters was determined by extracting the same syringes three times each by the same process. The percentage of extracted tungsten on first attempt compared with the sum of all three extractions was used to determine extraction efficiency. Target efficiency was constant 90% with initial extraction based on our requirements and practicality. Initial extractions with acid and water yielded significantly incomplete extraction of tungsten compared to base. Ammonium hydroxide was selected as extract solution due to its solubility for tungsten oxides and complexes, in comparison to the limited solubility of acidic solutions (14). There was no difference in extraction efficiency between 0.5% and 5.0% ammonium hydroxide concentrations.
Extracting the syringe barrels without the needle shield improved the extraction efficiency on average 7% compared to assembled syringes (barrel, plunger, and needle shield) using vacuum. Removal of the needle shield may allow more interaction between the syringe funnel area and extraction solvent, which may have resulted in higher tungsten extraction. In addition, pre-extraction step of moving the solvent in and out through the needle end allows complete solvent contact with the tungsten deposit area during extraction and maintains the funnel area free of air bubbles that form from agitation which might form a barrier between the extraction solvent and the funnel area. Sonication yielded on average 10% higher efficiency compared to incubation under the same conditions. Kinetic energy seemed to assist extraction more than thermal energy. Sonication extraction at 75 °C yielded on average 15% higher efficiency compared to room temperature sonication. Combination of kinetic and thermal extraction yielded the best results. Sonication or incubation at varying lengths of time did not result in any constant improvements in efficiency. We also constantly observed singular syringes within the same lot that did not extract as efficiently as others under the same conditions. There seems to be differences in how readily available the tungsten deposit area is to extraction, which affects efficiency.
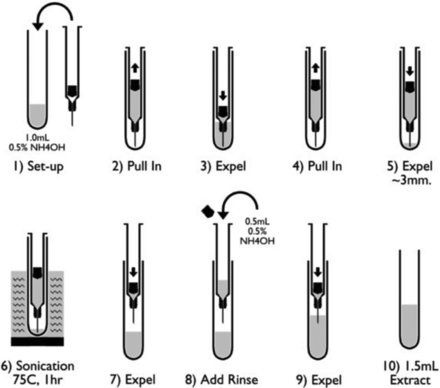
The determined extraction procedure is summarized in Figure 1. A syringe with a plunger manually inserted into the barrel and without a needle shield was placed in a commercially available glass tube (10 × 75 mm) filled with 1 mL of 0.5% ammonium hydroxide solution (pH 11). The solution was drawn into the barrel by pulling up the plunger, and the ammonium hydroxide solution was then expelled back into the glass tube. The solution was pulled back into the syringe a second time without any air gap and was partially expelled back into the tube, enough to cover approximately 3 mm of the needle. The tube containing the syringe was placed into a 75 °C pre-heated sonicator bath (VWR, Radnor, PA) for 1 h without the bath cover. The water level in the sonicator bath was maintained to the solution level within the syringe. After sonication, the tube with the syringe was removed from the bath and equilibrated to room temperature. The loss of extraction solution due to sonication was determined to be <1% of extraction volume (n = 10). The ammonium hydroxide solution was expelled from the syringe into the glass tube. The plunger was removed from the syringe, 0.5 mL of 0.5% ammonium hydroxide solution was added to the syringe, and this was expelled immediately into the initial 1 mL extraction solution through the needle. The total 1.5 mL of syringe extract was transferred into a 15 mL centrifuge tube (Corning, Corning, NY). An additional two extraction cycles were performed on the same barrel and glass tube by following the above steps. The second and third extracts were transferred into separate 15 mL centrifuge tubes. All the syringe extracts were vortexed thoroughly. Three syringe lots with low, middle, or high levels of tungsten were evaluated to determine process effectiveness. No leachable tungsten was observed from the glass tubes by themselves.
Tungsten extraction cycle of a glass syringe.
ICP/MS Method Development
The ICP/MS analytical technique was selected due to its sensitive and selective capability in measuring trace elemental tungsten in solutions. The ICP/MS method was developed using 0.5% ammonium hydroxide solution for sample diluents, standard diluents, and system wash, in order to maintain tungsten solubility. Iridium was selected as internal standard based on rarity and similar atomic weight to tungsten.
ICP/MS Parameters
A PerkinElmer/SCIEX Elan DRC II ICP/MS (Waltham, MA) equipped with an autosampler was optimized following the standard procedures recommended by the vendor. Masses were selectively monitored at 184 m/z (tungsten) and 193 m/z (iridium) with a dwell time of 50 ms. After each analysis, a 0.5% ammonium hydroxide solution was used to wash the system for 210 s.
Standard Preparation
Standards containing 0.1, 1.0, 5.0, 40.0, and 200.0 μg/L tungsten in 0.5% ammonium hydroxide and spiked to contain 50 μg/L iridium internal standard were used to determine a linear calibration curve. Each standard solution required a total volume of 5000 μL. The blank response was subtracted from the standards in determining the curve and in sample analysis.
Sample Extraction and Pooling for ICP-MS Tungsten Method Development
A large sample volume containing consistent tungsten concentration was required for ICP-MS method development purposes. Ten individual PFSs with attached needle shields were filled with 1 mL of 0.5% ammonium hydroxide, capped with plungers under vacuum, and sonicated at 75 °C for 1 h. The extracts were expelled and pooled. Each syringe was rinsed with 0.5 mL of 0.5% ammonium hydroxide and added to the pooled extracts. For ICPMS analysis, 300 μL of the syringe extract was further diluted 10 fold with 0.5% ammonium hydroxide and spiked to contain 50 μg/L Ir with a total volume of 3000 μL.
System Suitability
The ICP/MS instrument was optimized daily to meet the manufacturer's performance specifications (15).
Limit of Detection (LOD) and Quantification (LOQ)
Detection and quantification limits were evaluated using tungsten standards at 0.05 μg/L and 0.10 μg/L, respectively, in comparison to a 0.5% ammonium hydroxide control solution.
Limits were not evaluated in syringe extract solution, as the syringe extract contains endogenous levels of tungsten. To study the comparability between syringe extract solution and standard solvent 0.5% ammonium hydroxide in ICP/MS analysis, the matrix effect of the extract solution was studied and was shown to be negligible.
Selectivity
The selectivity of the ICP/MS detector for measuring tungsten isotope at 184 m/z was evaluated by analyzing osmium with isotope at 184 m/z and rhenium with isotope at 185 m/z. Each element at 10 μg/L in 0.5% ammonium hydroxide was analyzed for signal at 184 m/z and compared. The instrument was programmed to automatically differentiate between tungsten and osmium at 184 m/z based on isotopic distribution abundances.
The selectivity of the ICP/MS detector for measuring iridium at 193 m/z was evaluated by analyzing osmium with isotope at 192 m/z and platinum with isotope at 192/194 m/z. Each element at 10 μg/L in 0.5% ammonium hydroxide was analyzed for signal at 193 m/z and compared.
Accuracy
The accuracy of the tungsten quantification was evaluated by analyzing blanks (50 μg/L Ir in 0.5% ammonium hydroxide) spiked to contain 0, 0.5, 10, or 180 μg/L tungsten and determining the observed concentrations against a calibration curve. Comparison of the observed tungsten concentration to the theoretical would demonstrate accuracy.
Matrix Effect
The matrix effect of syringe extract was evaluated by analyzing un-spiked syringe extract and syringe extract sample spiked with 1, 10, or 100 μg/L. The recovery of the tungsten in the spiked samples would demonstrate any matrix effect.
Precision
The precision of tungsten quantification was evaluated by analyzing un-spiked syringe extract and syringe extract sample spiked with 1, 10, and 100 μg/L. For intraday precision, the samples were analyzed five times in one day. For-day to-day precision, the samples were analyzed on 3 separate days.
Linearity
The linearity of tungsten quantification was evaluated by analyzing the slope, intercept, and correlation coefficient (R) of the W/Ir calibration curve run over 3 days.
Carryover
The carryover of tungsten and iridium after a 210 s wash with 0.5% ammonium hydroxide was evaluated. A blank run after 200 μg/L tungsten would demonstrate any carryover of tungsten in 0.5% ammonium hydroxide. 0.5% ammonium hydroxide run after a blank would demonstrate any carryover of iridium in 0.5% ammonium hydroxide.
Syringe extract sample run after syringe extract spiked with 200 μg/L tungsten would demonstrate any carryover of tungsten in syringe extract. 0.5% ammonium hydroxide run after syringe extract sample would demonstrate any carryover of iridium in syringe extract.
Stability
The stability of syringe extract for tungsten quantification was evaluated by analyzing the same syringe extract over 3 consecutive days stored at room temperature and calculating the relative standard deviation (RSD) of the observed tungsten levels over 3 days.
Robustness
The robustness of tungsten quantification would be evaluated for variability in ammonium hydroxide concentration. Ammonium hydroxide was prepared at ±10% of 0.50%, or 0.45 and 0.55%. Standards were prepared with 0.45, 0.5, or 0.55% ammonium hydroxide, respectively, and run on ICP/MS. The three concentration of ammonium hydroxide was spiked with 0, 1, 10, or 100 μg/L tungsten and analyzed. Syringe extract samples was prepared with 0.45, 0.50, or 0.55% ammonium hydroxide and analyzed. The precision of the observed calibration curve parameters, spiked samples, and syringe extract samples were used to demonstrate the robustness of the measurements as a function of the ammonium hydroxide concentration.
III. Results and Discussion
LOD and LOQ
The detection and quantification limits were evaluated by analyzing a control (0.5% ammonium hydroxide) and 0.05 or 0.1 μg/L tungsten in 0.5% ammonium hydroxide. The 0.05 and 0.1 μg/L tungsten standards had signal response counts 40 and 80 times greater, respectively, than those observed from the control. The detection and quantification limits at 0.05 and 0.1 μg/L tungsten were acceptable based on the signal-to-noise ratio greater than 3 and 10, respectively (16).
Selectivity
Selectivity of the ICP/MS detector for measuring tungsten at 184 m/z and iridium at 193 m/z was evaluated by analyzing a control (0.5% ammonium hydroxide), tungsten, osmium, rhenium, and platinum at 10 μg/L in 0.5% ammonium hydroxide. Measurable amounts of tungsten at 184 m/z and iridium at 193 m/z were not observed in 10 μg/L solutions of osmium, rhenium, and platinum. The selectivity of the ICP/MS for tungsten at 184 m/z and iridium at 193 m/z was acceptable based on identification of positive and negative results by the instrument (16).
Syringe extract did not contain detectable levels of endogenous iridium.
Calibration Curve Linearity
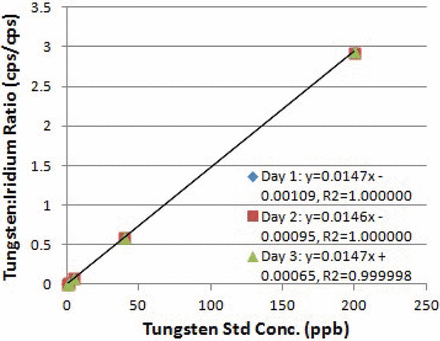
Calibration curves (0.1–200.0 μg/L W) were prepared daily over 3 days, and a linear regression analysis was performed for the three curves (Figure 2). A linear relationship between tungsten concentration (μg/L) and cps ratio of W/Ir between 0.1 and 200.0 μg/L W was observed [correlation coefficient (R) = 1.0000 ± 0.0000]. The slope and intercept were 0.0147 ± 0.0001 and 0.0008 ± 0.0003, respectively. Both the slope and R had an RSD (n = 3) of <1%.
Linerarity of tungsten quantification over 3 days.
Carryover
Carryover of tungsten and iridium was evaluated by analyzing a blank (0.5% ammonium hydroxide) after the 200 μg/L W standard and a 50 μg/L Ir 0.5% ammonium hydroxide solution, respectively. No detectable level of tungsten and iridium were observed in the 0.5% ammonium hydroxide blanks that immediately followed the 200 μg/L W and 50 μg/L Ir solutions, respectively. Carryover from syringe extracts spiked with 200 μg/L tungsten and 50 μg/L iridium were also evaluated. Analysis of blank solution following the syringe extracts did not contain observable levels of tungsten and iridium. The carryover of tungsten and iridium were characterized as negligible and thus acceptable.
Accuracy
Tungsten concentration in 0.5% ammonium hydroxide solutions spiked with 0, 0.5, 10.0, and 180.0 μg/L tungsten with 50 μg/L Ir were determined using a calibration curve. Accuracies of tungsten concentrations in all samples were within 1% of the expected concentrations (Table I). The method accuracy for elemental tungsten was acceptable (16).
Accuracy of Elemental Tungsten Quantification
Matrix Effect
Syringe extracts spiked with 0, 1.0, 10.0, and 100.0 μg/L tungsten had recoveries ranging between 90 and 96% at each concentration level (Table II). This suggests that the effect of the syringe extract matrix was comparably negligible to that of standard solvent 0.5% ammonium hydroxide, justifying the LOD and LOQ analyses performed in 0.5% ammonium hydroxide instead of syringe extract.
Matrix Effect of Syringe Extract on Elemental Tungsten Quantification
Precision
Intraday precision was evaluated by analyzing syringe extract samples spiked with 0, 1.0, 10.0, and 100.0 μg/L tungsten (Table III). Five replicates per spiked concentration were evaluated. The determined tungsten at each spike concentration had an RSD (n = 5) of ≤1%. The method intraday precision for elemental tungsten was acceptable (16).
Intraday Precision of Elemental Tungsten Quantification
Interday precision was evaluated by preparing and analyzing syringe extract samples spiked with 0, 1.0, 10.0, or 100.0 μg/L tungsten on 3 different days (Table IV). The determined tungsten at each spike concentration had an RSD of ≤1%. Interday precision for elemental tungsten was acceptable based on low RSD for analyzed samples (16).
Interday Precision of Elemental Tungsten Quantification
Stability
Stability of syringe extract was evaluated by analyzing the same syringe extract that was stored at room temperature for 3 consecutive days. The concentration of tungsten in the extract analyzed over 3 days was 6.2 ± 0.1 μg/L W with an RSD (n = 3) of <2%. This demonstrated that the syringe extract was stable up to 3 days at room temperature.
Robustness
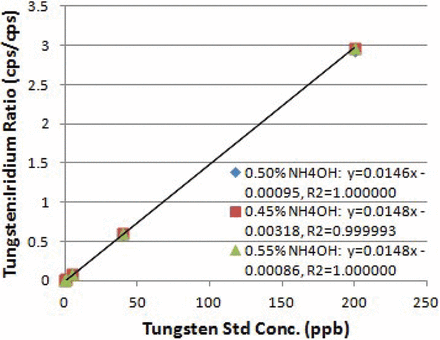
Calibration curve, standards, and syringe extracts in 0.50 ± 0.05% ammonium hydroxide were prepared and analyzed for tungsten (Figure 3). The three curves in 0.45, 0.50, and 0.55% ammonium hydroxide had R values of 1.0000 and slopes of 0.0147 ± 0.0001. The accuracies at 0.5, 10.0, and 180.0 μg/L W were within 1% of the expected concentrations (Table V). Syringe extract diluted 10 fold with 0.5% ammonium hydroxide contained 6.1 μg/L W. Extracts prepared from 0.45 and 0.55% ammonium hydroxide contained 6.5 and 6.4 μg/L W, respectively, <7% difference from the 0.5% ammonium hydroxide extract. The robustness of ammonium hydroxide concentration was acceptable (16) up to ±10% of the target concentration of 0.5%.
Linerarity of tungsten quantification with varying ammonium hydroxide concentration.
Robustness of Ammonium Hydroxide Concentration on Accuracy for Elemental Tungsten Quantification
Extracted Tungsten Mass and Efficiency of 30 PFSs from Three Manufacture Lots
Thirty PFSs from three lots were extracted (according to Figure 1) three times each and each extract analyzed separately by ICPMS. Observed extract concentration was converted to mass (ng) and the extraction efficiency (%) was calculated for each extraction compared to the total of all three extracts per syringe (Table VI). Syringe Lot 1 (low tungsten) had on average 28.0 ng of extracted tungsten with a range from 9.8 to 53.1 ng. Extraction efficiency for Lot 1 was on average 75.2% for the first extraction and 18.2% for the second extraction. Other than the one syringe with very low tungsten (13 ng), Lot 1 samples had total extraction efficiency greater than 90% with the combination of first and second extraction rates. Syringe Lot 2 (middle tungsten) had on average 189.4 ng of extracted tungsten with a range from 56.7 to 302.9 ng. Extraction efficiency for Lot 2 was on average 92.6% for the first extraction and 5.7% for the second extraction. All Lot 2 samples had total extraction efficiency greater than 91% with the combination of first and second extraction rates. Syringe Lot 3 (high tungsten) had on average 631.9 ng of extracted tungsten with a range from 237.6 to 1336.8 ppb. Extraction efficiency for Lot 3 was on average 93.3% for the first extraction and 4.9% for the second extraction. All Lot 3 samples had total extraction efficiency greater than 94% with the combination of first and second extraction rates. All syringes with tungsten greater than 14 ng had total extraction efficiency greater than 90% with the combination of first and second extraction rates.
Extracted Tungsten Mass (ng) and Efficiency (%) from Glass Prefilled Syringes
IV. Conclusion
The ICP/MS method is suitable for determining extracted tungsten from PFSs between 0.1 and 200.0 μg/L W based on acceptable limit of detection/quantification, accuracy, matrix effect, precision, linearity, carryover, stability, and robustness. Two extraction cycles per syringe using 0.5% ammonium hydroxide solution, heat (75 °C), and sonication seems adequate in extracting >90% of tungsten residues. The method should enable screening syringe lots for residual tungsten.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank Ping Yeh, Ronald Forster, and Bruce Eu for their guidance and support.
- © PDA, Inc. 2013