Abstract
Delamination of flakes in glass containers used for primary drug packaging has become a serious quality concern in recent years. Because glass delamination typically occurs weeks/months after filling and there are a variety of container, processing and drug formulation factors that contribute to glass delamination, it is recommended according to USP <1660> (1) to conduct suitable container/drug product compatibility tests. Such predictive studies should give results that allow a graduated assessment for increasing risk of delamination that can be used to detect early stages of this phenomenon and to help to select appropriate container/formulation systems to proactively prevent delamination instead of just monitoring for the presence/absence of flakes. This work demonstrates the capability of a container compatibility testing approach by determining the impact of three different model buffer/solution systems (citrate, phosphate and sodium bicarbonate) with the delamination behavior of two different types of 2 mL glass vials, standard quality and delamination controlled quality. Vials of each type were filled and stored up to 48 weeks at 40 °C. Using a USP <1660>–compatible package of different analytical methods clearly demonstrates the significant influence of both vial quality and chemistry of the content on glass delamination propensity.
LAY ABSTRACT The detachment of flakes from the inner surface of a glass container—also referred to as delamination—has become a serious quality concern over the last years for the pharmaceutical industry. Chapter <1660> of the United Stated Pharmacopeia therefore recommends performing predictive screening studies with the drug formulation and the glass container to evaluate the risk of delamination in an early stage of the drug development. Predictive screening studies have been performed with three different representative buffer/solution and glass vials of two different quality steps (standard and delamination controlled). The results demonstrate the capability of container compatibility testing and the influence of vial quality and chemistry of the content on glass delamination propensity.
Introduction
The observation of visible flakes in pharmaceutical parenteral products is the last and obvious step associated with the glass delamination process. This phenomenon is the result of a multifacetted interaction between the pharmaceutical formulation and the interior surface of the glass container. Once these flakes are observed in a commercial product, it is too late to correct the problem and a recall is unavoidable. USP <1660> (1) recommends conducting suitable container/drug product compatibility tests. A reasonable number of publications in recent years were focused on collecting data and information about the root causes (2⇓⇓⇓⇓–7). As a result the principle mechanisms are understood and it became clear that the entire delamination process is complex with multiple influencing factors. At the end it is an interplay between different variables comprising container properties, the chemical composition of the drug formulation, as well as the filling and storage parameters.
This situation clearly demonstrates from the pharmaceutical packaging side the absolute necessity to predict the risk of delamination instead of being blind until the lagging indicator “glass flakes” appears and requires the primary packaging manufacturers to have alternative container solutions commercially available. The work done to date has allowed substantial progress in both testing and container development. On one side predictive pre-delamination features can be found by appropriate testing well before flakes are observed. This was the basis for the 2013 USP <1660> guidance “Evaluation of the Inner Surface Durability of Glass Containers” (1) giving some general advice for analyzing the interaction mechanisms between the pharmaceutical product and glass container with emphasis on glass delamination detection. Over the last years SCHOTT pharma services has refined a delamination screening package combining several analytical techniques that is aligned with USP <1660> and successfully applied in hundreds of studies for a large range of drugs (5). On the other side advanced development in the manufacturing process of glass containers enabled a significant reduction in delamination propensity. This progress was achieved by evolutions in the converting process in combination with an appropriate test method for the rapid monitoring of the production runs as described by the authors elsewhere (7). In this paper we describe the application of predictive delamination testing using pharma-relevant model buffer/solution systems for the evaluation of vials with different delamination risk (delamination controlled quality versus standard quality).
Materials, Methods and Equipment
Materials
Two different vial types with a nominal filling volume of 2 mL were produced on state-of-the-art converting lines using glass tubes made of FIOLAX® (Type I B borosilicate glass). The first type of vials (delamination controlled quality) was formed applying an optimized set up in combination with a monitoring test procedure named “Quicktest”. The creation of zones with increased vulnerability in the wall near bottom area is thereby avoided based on a validated threshold value (Quicktest limit value) (7).
For the second type of vials (standard quality) a setup has been applied to produce vials in accordance with regulatory requirements like USP, Ph. Eur. and JP but exceeding the limits defined by the Quicktest. The Quicktest is a quantitative measure for an increased vulnerability in the wall near bottom area, which can increase the risk for a delamination recall (8).
Prior to filling, storage, and analyses, all vials were cleaned in two steps with tap water (first) and purified water (second) and then cautiously labeled with a waterproof pencil to avoid the creation of splinters. The vials were then filled with three different buffers/solutions (50 vials per type) with the respective filling mediums as described below:
Citrate buffer 10 mMol: pH 6.0 with 150 mMol NaCl and 0.005% Tween 20
Phosphate buffer 10 mMol: pH 7.0 with 150 mMol NaCl and 0.005% Tween 20
Sodium bicarbonate solution: 8.4% NaHCO3 (starting with about pH 8)
After capping the filled vials with Westar RS B2-40 Fluro-Tec stoppers, the vials were stored in an oven in an upright position at 40 °C; the relative humidity was not controlled. The study design consisted of storage with five time points: 15 h, 4 weeks, 12 weeks, 24 weeks, and 48 weeks storage. For each time point, vial type, and filling solution 10 vials were used (300 vials in total). The storage at 40 °C for up to 48 weeks was selected to be in accordance with the temperature and time period instructions given in the ICH Q1A(R2) guideline for accelerated studies (40 °C and 6 months minimum).
Methods
The investigations with respect to indications for glass delamination were conducted in alignment with the recommendations of USP <1660> as described below. Additional information is given in Haines et al. (5).
Visual inspection by eye and magnifying video camera with respect to the presence/absence of “flake-like” particles (in-house methods). The vials were rotated gently to swirl up potential particles. Subsequently a visual inspection of the vials was performed under illumination with a cold light source. In a next step, the rotation was repeated and the inspection was done with a camera system. This in-house method applied is designed to visualize flakes and is more sensitive than the pharmacopeia standards (e.g. USP <790>) (9).
Optical inspection by stereo-microscopy with extended depth of focus to qualitatively determine if there are any indications for reaction zones or glass attack present on the vial interior surface. The optical inspection by means of stereo-microscopy is focused on the wall near bottom area. Within this area the appearance was classified with respect to coloration and light scattering. Light scattering and coloration indicate a changed inner surface. The coloration is caused by an interference effect generated by an altered layer at the interior surface with a refraction behavior different to the bulk glass, which is an indication of a delamination risk. The minimum thickness that could be seen as coloration depends on the refraction index of the altered layer.
Selection of the two “worst” vials per sample type for subsequent scanning electron microscopy (SEM) surface analyses.
SEM cross-section analyses at the vial interior surface in the vulnerable areas of the two vials as identified by stereo-microscopy. The analyses were carried out in the wall near bottom and mid-body area (reference area). This investigation reveals the presence or absence of a potential reaction zone.
Inductively coupled plasma optical emission/mass spectroscopy (ICP-OES/MS) analyses of the contents pooled from the vials of each sample type to quantitatively determine the concentration of “glass” elements leached into the solution for 4 elements (Si, B, Al and Ca) to ascertain if the amount found reveals a pronounced chemical attack. This investigation helps to determine the corrosion mechanism.
Equipment
Stereo-Microscopy:
For the optical inspection of the vulnerable area in the heel region of the containers, a stereo-microscope (Zeiss Discovery V20) equipped with a camera system was used.
Scanning Electron Microscopy (SEM):
The SEM (LEO 1550 including EDS) was used to characterize the morphology of surfaces and cross-sections and to analyze the particles find in filtrate residue.
Inductively Coupled Plasma Mass Spectroscopy (ICP-MS, ICP-OES):
For the measurement of the concentration of typical “glass” elements, an Agilent ICP-OES 725 series (Si) and an Agilent ICP-MS 7500ce (B, Al, and Ca) was in place.
Results and Discussion
The first visual inspection found “flake-like” particles present especially for the vials filled with phosphate buffer and sodium bicarbonate solution stored for 24 and 48 weeks.
After emptying the vials, an optical investigation was conducted in the wall near bottom and mid-body regions using a stereo-microscope. For tubular vials the delamination process starts in most cases in the wall near bottom area. The special vulnerability of this area is a result of the converting process and described in Rupertus et al. (7). When observed at the mid-body, this indicates a drug formulation/glass chemistry incompatibility. The inspections were focused on the presence/absence of a coloration band that when observed indicates a layer at the interior surface with a modified composition (reaction zone) as a result of glass corrosion. At the end of the study (time point 48 weeks) no coloration was found for the delamination controlled quality vials. In contrast the standard quality vials filled with sodium bicarbonate (all 10 vials) and phosphate buffer (6 of 10 vials) featured weak to medium coloration, while no coloration was present for the vials filled with citrate buffer.
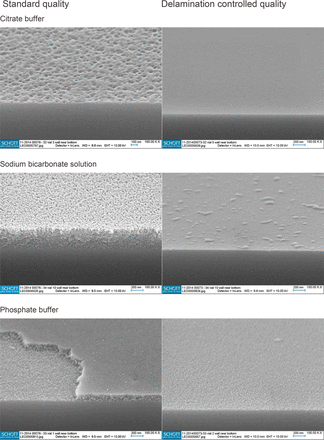
On the basis of the results derived from the stereo-microscopy the most attacked or altered areas of the “worst” vials were marked for SEM cross-section analyses. By fracturing the vials the respective interior surface became accessible. Two areas of each selected vial were prepared in this way and characterized, one from the wall near bottom and a reference from the mid-body. The resulting micrographs demonstrate the detailed morphology of the interior surface with many distinct features. The micrographs found in the more vulnerable wall near bottom area after 24 weeks storage are depicted for both vial types in Figure 1. For a better comparison the presence of the following characteristic features is summarized in Tables I and II:
Micro-roughness of the interior surface
Reaction zones, found as a thin layer with different morphology (e.g. increased porosity)
Delaminated areas where a part of the reaction zone was detached
SEM cross-section micrographs from the wall near bottom area after 24 weeks storage filled with different buffer systems at 40 °C.
Characteristic Features from SEM-Analyses for Standard Quality for 24 Weeks at 40 °C
Characteristic Features from SEM Analyses for Delamination Controlled Quality for 24 Weeks at 40 °C
Regarding the criticality, we make the following categorizations. When delaminated areas were observed a delamination has already taken place, while the appearance of a reaction zone can be seen as an early indicator (i.e. pre-delamination feature). Micro-roughness as a single feature gives a hint only for a corrosion (i.e. homogenous dissolution) in general with no special risk for delamination.
The standard quality vials filled with citrate buffer exhibited a significant micro-roughness in the wall near bottom area as can be seen in Figure 1. By contrast the surface of the vials with delamination controlled quality appeared smooth within the same area. No delamination or early indicators were observed for both vials types.
The micrographs taken after storage with sodium bicarbonate solution revealed a pronounced reaction zone with a thickness up to 360 nm only in the vulnerable area of the standard quality vials. This reaction zone showed a porous morphology with a rough surface.
Delamination was confirmed by the observation of delaminated areas in the wall near bottom area of standard quality vials filled with phosphate buffer. Pieces of the reaction zones are detached from the interior surface, leaving behind sharply defined borders that can be seen in the SEM images. Reaction zones with a thickness up to 140 nm (standard quality) and 30 nm (delamination controlled quality) occurred together with micro-roughness for both vials types after storage for 24 weeks with phosphate buffer.
Supplementary to the characterization of the vials itself, the concentrations of the typical “glass” elements Si, B, Al and Ca dissolved from the vials were determined using ICP measurements of the pooled contents of 10 vials at several time points. Although this information does not directly confirm glass delamination, it is very helpful to verify the glass attack mechanism taking place (i.e. homogenous dissolution, selective dissolution, or in between) at each time point prior to the observation of glass flakes. The data found after 24 weeks storage is listed in Tables III and IV.
ICP Analyses of “Glass” Elements for Standard Quality for 24 Weeks at 40 °C
ICP Analyses of “Glass” Elements for Delamination Controlled Quality for 24 Weeks at 40 °C
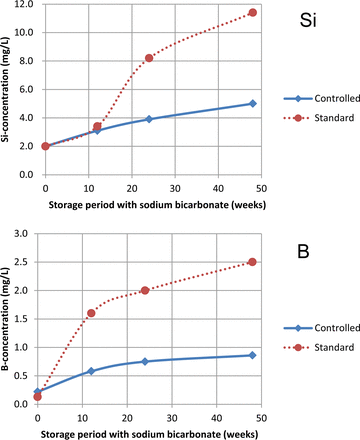
The ICP results of the citrate buffer were quite similar for both vials types with silicon concentrations of about 20 mg/L and boron concentrations of around 2 mg/L. This Si/B ratio of about 10 corresponds fairly well to the Si and B amounts (weight) of Fiolax (10) indicating a more or less homogenous dissolution of the glass, which was expected on the basis of existing data (11, 12). This contrasts with the situation found for the sodium bicarbonate solution. Significantly higher concentrations of silicon and boron with lower concentrations of aluminum were found for the standard quality compared to controlled quality vials. As depicted in Figure 2, there is a steep increase in the boron concentration in the first 12 weeks of storage, while a pronounced rise of the silicon values was seen delayed between 12 and 24 weeks storage for the standard vials. The Si/B ratios in the range of 4 (standard) and 5 (controlled) point to a selective dissolution with a preferential release of boron. The composition of a near surface layer in the vulnerable areas in the wall near bottom area where delamination usually starts is modified in a way that boron can be dissolved more easily (7). Therefore, the observation of low Si/B ratios can be seen as an early indicator for delamination and were used together with the results from the other analyses for risk assessment. A third interaction process between the glass and the content is disclosed by taking a close look at the values derived for the phosphate buffer after 24 weeks storage (Tables III and IV). There is also a big difference between the two vial types regarding the amounts of silicon, boron and calcium with elevated levels for the standard quality, which seems similar to the situation with the sodium bicarbonate. On the other hand the Si/B ratio found for the phosphate buffer is in the range of 10 and this is similar to the results with citrate buffer. In addition, very low concentrations of aluminum (<0.1 mg/L) were determined for both vial types. The mechanism at work that retained the aluminum from going into solution has also been reported by others (13).
Concentrations of silicon and boron after storage with sodium bicarbonate at 40 °C for different time periods.
By combining the results from the set of analytical methods, a better insight into the interaction process between the different buffers/solutions and the interior surface of the vials becomes possible. If this interaction is dominated by homogeneous dissolution no delamination will appear but a difference in composition localized at the wall near bottom area can lead to the roughening of the surface, which is the mechanism found for the citrate buffer. Roughening of the surface only is typically labeled as glass attack. As a consequence the selected citrate buffer is not suitable to predict the risk for delamination under the conditions chosen in this study. The situation is totally different when preferential dissolution/leaching of a main glass component (e.g. boron or sodium) gains importance as we have seen for the sodium bicarbonate solution. This creates a leached layer that can be visualized by SEM as a reaction zone with a porous morphology or as a coloration that can be seen with the stereo-microscope. Such layers can detach and generate flakes with a glass-like composition. Another mechanism that needs to be considered is the reaction between components of the content with glass elements that results in solid compounds. Especially for phosphates it was shown that aluminophosphate-rich layers can build at the interior glass surface (5, 13) and release in fragments that are poorly soluble. Our findings of very low concentrations of aluminum (< 0.1 mg/L) together with the reaction zones in the wall near bottom area for the phosphate buffer fit to this interaction behavior. The situation could change if the vials with phosphate buffer at pH 7 went through temperature treatment similar to terminal sterilization. In this case a significant concentration of dissolved aluminum was found by Ogawa et al. (13), which decreases with further storage at lower temperatures of 5 °C, while Al-rich particles are built (precipitation).
In summary, this work found that an increased delamination risk is present for solutions causing a selective dissolution or the formation of solid compound layers. Direct head-to-head comparison of the two vial types demonstrated that glass attack can be reduced by well-adjusted steps of vial manufacturing. The controlled quality vials demonstrated higher durability especially in the critical wall near bottom area, which was strikingly observed for sodium bicarbonate solution and phosphate buffer. This trend can be seen in more detail by categorizing the analytical results with respect to the criticality after 24 weeks in Table V. Delamination was confirmed by the presence of delaminated areas (SEM) for phosphate buffer stored in standard quality vials. A lower delamination risk was found on the level of early indicators for standard quality filled with sodium bicarbonate (reaction zone and coloration) and for controlled quality vials stored with phosphate buffer (small reaction zone).
Classification of the Different Analytical Results with Regards to the Criticality for 24 Weeks at 40 °C
For none of the vial types neither early indicators nor delamination was observed with citrate buffer. However, the high silicon concentration observed for both vial types and the micro-roughness (standard quality only) gave evidence for the homogenous dissolution caused by glass attack by the citrate buffer.
Conclusion
A combination of different analytical methods was applied to evaluate the impact of citrate buffer, phosphate buffer, and sodium bicarbonate solution for the delamination propensity of two types of vials with different corrosion behavior. The reported results demonstrate that the USP <1660>–compatible screening protocol is suitable to give graduated levels of predictive information (e.g. glass attack, indicators for delamination, delamination confirmed) which is in general needed for a risk assessment for container/formulation compatibility and suitability, rather than looking only for glass flakes. Even for various interaction mechanisms like homogenous and selective dissolution or the formation of compound layers, a distinction between different quality levels of the vials with regards to delamination can be determined. Nevertheless, it must be clear that such studies should be conducted with the relevant drug or placebo solution and considering lot-to-lot variations. In addition, the results demonstrate the potential to reduce the vulnerably of tubular vials especially in the critical areas where delamination starts first by using special converting procedures in combination with suitable monitoring tests during vial production.
Conflict of Interest Declaration
The authors declare that they do not have any financial or non-financial competing interests related to the content of the article.
- © PDA, Inc. 2016