Abstract
Considerable resources are spent within the biopharmaceutical industry to perform viral clearance studies, which are conducted for widely used unit operations that are known to have robust and effective retrovirus clearance capability. The collaborative analysis from the members of the BioPhorum Development Group Viral Clearance Working Team considers two common virus reduction steps in biopharmaceutical processes: low-pH viral inactivation and viral filtration. Analysis included eight parameters for viral inactivation and nine for viral filtration. The extensive data set presented in this paper provides the industry with a reference point for establishing robust processes in addition to other protocols available in the literature (e.g., ASTM Std. E2888-12 for low-pH inactivation). In addition, it identifies points of weakness in the existing data set and instructs the design and interpretation of future studies. Included is an abundance of data that would have been difficult to generate individually but collectively will help support modular viral clearance claims.
Background
Viral safety is a key concern in the growing field of biotechnology products derived from animal cell lines and intended for therapeutic use. Rodent cell lines may contain endogenous retroviruses or retrovirus-like particles as measured by transmission electron microscopy (1). Furthermore, cell cultures may be contaminated by adventitious viruses introduced through raw materials or handling (2, 3). To ensure the safety of human therapeutics, there is a regulatory requirement to demonstrate the ability of a purification process for a recombinant product to remove or inactivate a wide variety of potential viral contaminants in investigational or commercial products (4⇓–6). It is commonly accepted that effective steps are those delivering >4 log10 reduction factor [LRF; LRF is equivalent to LRV (log10 reduction value)] of virus, although steps providing >1 LRF may contribute to overall removal (7).
A risk-based approach to viral clearance, taking into consideration historical process knowledge, could reduce the testing scope for well characterized steps (8). However, small and homogenous data sets possessed by individual firms using a purification platform may be insufficient to support this effort (9). A resolution to the problem of homogenous data sets is sharing viral clearance data across firms. Ten firms have collaborated through the Biophorum Development Group (BPDG) to share and compare viral clearance values obtained by manufacturing steps to define a scientifically sound strategy for validating future recombinant biotechnology products. Following an initial benchmarking activity, low-pH inactivation and viral filtration steps were selected as robust and effective steps that could benefit from a risk-based approach to validation (10⇓–12).
This report presents the results from a rigorous statistical data analysis and comparison across ten firms and proposes how the findings may support a risk-based approach to validation, including modular viral clearance claims.
Materials and Methods
Data Source
A database was constructed using blinded survey responses from ten biotechnology companies including: AbbVie, Inc., Bayer Corporation, Biogen, Bristol-Myers Squibb, GlaxoSmithKline plc, ImmunoGen, Inc., Merck, Sharp and Dohme, Inc., Novartis International AG, Regeneron Pharmaceuticals, Inc., and Shire plc. Each firm contributed retrospective good laboratory practice–compliant viral clearance data from individual experiments evaluating low-pH viral inactivation and viral filtration. Virus infectivity was determined using cell-based assays with limit of detection determined by application of Poisson distribution when virus infectivity was reduced to nondetectable levels (6). Submitted data were compiled and blinded by a BPDG Viral Clearance Working Group facilitator and imported into SAS JMP® v11.1.1 for review and statistical analysis.
Low-pH Viral Inactivation
A total of 162 individual viral inactivation experiments were reported for products, which included Immunoglobulin G 1 (IgG1) (102/162), IgG4 (26/162), and other (34/162). The “other” category includes products that are not restricted to IgG1 or IgG4 monoclonal antibodies, such as non-Fc fusion recombinant proteins, nondisclosed recombinant proteins, and IgG2 isotype monoclonal antibodies. Three enveloped model viruses were used in spiking studies: xenotropic murine leukemia virus (XMuLV) (138/162), Suid herpesvirus (SuHV-1) (21/162), and herpes simplex virus (HSV-1) (3/162). Table I summarizes the physical characteristics of the viruses used for spiking studies.
Viruses Used in Spiking Studies
The database included pH from 3.40–3.95, which is wider than the range covered by the ASTM standard E2888-12 but did not include conditions expected to show insufficient viral clearance (e.g., pH >4.4) (13). Kinetics of inactivation were reported for all experiments. Temperature was categorized into levels: 2–8 °C, 15 ± 1 °C, and 16+ °C. TCID50 (the tissue culture infectious dose that infects 50% of the cell monolayers challenged with the defined inoculum) and plaque assays were considered equivalent for the purposes of estimating total virus load, resulting in a range from 4.74 to 9.16 log10. When protein concentration was reported as a range (e.g., 7–10 g/L), the average was calculated, because protein concentration is not anticipated to have a significant impact on LRF for a small range, resulting in an overall range of 3.5–28.8 g/L. Buffer systems included acetate, citrate, and other. The “other” category included nondisclosed buffers and others present in low numbers (e.g., N < 12), such as formate, glycine, HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], succinate, and phosphate.
Viral Filtration
In total, 392 individual viral filtration experiments were reported. Filter types were limited to small virus filters categorized into polyethersulfone (PES) (N = 150/392 experiments) and regenerated cellulose (RC) (N = 242/392 experiments) materials (11). These two materials of construction included four filter brands and were treated separately due to geometry (i.e., flat sheet PES versus hollow fiber RC), distinct operating pressures, and published literature implicating potentially different failure modes for parvovirus (14, 15). For PES filters, total virus load ranged 3.27–9.08 log10, pH 4.6–8.5, volumetric loading 127–2742 L/m2, protein concentration 1.5–22 g/L, mass loading 271–7500 g/m2, and operating pressure 14–35 psi. For RC filters, total virus load ranged 3.76–10.22 log10, pH 5.0–8.5, volumetric loading 73–1321 L/m2, protein concentration 1.6–25 g/L, mass loading 267–6935 g/m2, and operating pressure 10–14.5 psi.
Proteins included IgG1 mAb (207/392), IgG4 mAb (72/392), and other (113/392). The “other” category includes non-Fc fusion recombinant proteins, nondisclosed recombinant proteins, and IgG2 isotype. Model viruses used in spiking studies ranged from large enveloped viruses [SuHV-1, HSV-1, XMuLV, and amphotropic murine leukemia virus (AMuLV) in 192/392 experiments] to nonenveloped parvoviruses (PPV and MMV in 148/392 experiments). Intermediate size viruses [Reovirus Type3 (Reo3) and encephalomyocarditis virus (EMCV) in 52/392 experiments] make up the remainder.
Results and Discussion
Low-pH Viral Inactivation
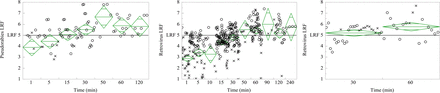
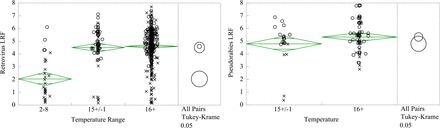
Virus inactivation (LRF) has been plotted versus time for retrovirus and herpesvirus based on adherence to ASTM E2888-12 as shown in Figure 1. The claims of ASTM E2888-12 are supported by this data set for both viruses; mean retrovirus clearance is 5.43 ± 0.85 LRF, and for cases with less than 5.0 LRF, reported virus infectivity is reduced below the limit of detection (LOD) within the limits of the standard. Figure 1 shows retrovirus experiments that do not conform to ASTM E2888-12 achieve mean clearance of 4.79 ± 1.03 after 30 min. Figure 2 shows that low temperature reduces inactivation, with 2–8 °C resulting in lower clearance than 15 °C (2.05 ± 1.63 versus 4.52 ± 1.53 LRF, P < 0.0001), consistent with thermodynamics of viral inactivation. Figure 3 shows a contribution from pH and virus load (P <0.001), where pH affects capsid denaturation and virus load limits the maximum reportable clearance (6, 16, 17). Virus load and pH are correlated (R = 0.48), and the effects of these individual factors cannot be elucidated.
Mean clearance of virus during low-pH inactivation as a function of time for pseudorabies (left), retrovirus not conforming to ASTM E2888-12 (center), and retrovirus conforming to E2888-12 (right). (○) indicates virus infectivity reduced below detection limits. (×) indicates infectious virus detected.
One-way analysis of virus LRF versus temperature for retrovirus (left) and pseudorabies (right). (○) indicates virus infectivity reduced below detection limits. (×) indicates infectious virus detected. Comparison of mean LRF (95% confidence) shows no statistically significant difference between 15 ± 1 °C and 16+ °C, while clearance at 2–8 °C is significantly lower (P < 0.0001).
One-way analysis of retrovirus LRF after 30 minutes versus protein concentration (left), pH (center), and virus load (right). (○) indicates virus infectivity reduced below detection limits. (×) indicates infectious virus detected. The mean is presented as a bold black line while the least squares regression is gray (95% confidence). There is a statistically significant trend for pH and virus load (P < 0.001), while protein concentration is not significant (P = 0.41).
Inactivation kinetics are faster for pseudorabies than retrovirus, with SuHV-1 clearance of 4.25 ± 1.93 LRF after 1 min and 5.51 ± 1.18 LRF after 30 min despite pH >3.60 in all experiments. SuHV-1 infectivity is reduced below the LOD after 30 minutes in 18/19 cases. The result is surprising, as pseudorabies is generally regarded as displaying medium resistance to physico-chemical treatments compared to low resistance for rodent retrovirus (6). One gap identified was that the database does not include examples of SuHV-1 inactivation at 2–8 °C. The impact of temperature on inactivation kinetics for this virus could not be assessed. Inactivation of HSV-1 was less robust, and a single experiment at pH 3.8 resulted in less than 3 LRF after 30 min, suggesting greater sensitivity to pH for that virus outside the limits of ASTM E2888-12.
Viral Filtration
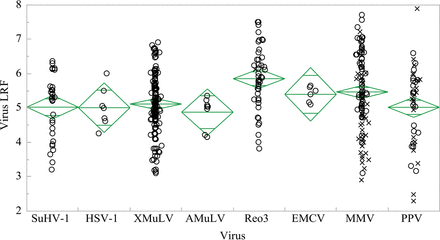
Virus removal has been plotted as shown in Figure 4. Mean clearance exceeded 4 LRF for all model viruses as expected for small virus filters. Infectivity of large enveloped viruses SuHV-1, HSV-1, XMuLV, and AMuLV was reduced below the LOD in all cases (198/198). Reo3 infectivity was reduced below the LOD in 45/46 tests and demonstrated 5.86 ± 0.91 log10 average clearance. Clearance was 6.12 LRF in the experiment with residual Reo3 infectivity. Mean Reo3 removal in two replicates was 0.2 LRF higher than mean porcine parvovirus (PPV) clearance for the same recombinant protein, further supporting the use of parvovirus as worst case.
Average log10 reduction for model viruses by small-virus filter class. (○) indicates virus infectivity reduced below detection limits. (×) indicates infectious virus detected.
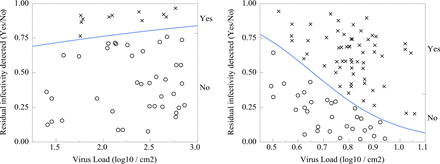
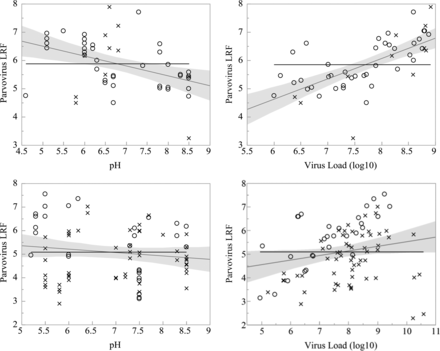
The smallest viruses, murine parvovirus (MMV) and PPV, demonstrated mean clearance of 5.47 ± 1.13 log10 and 5.02 ± 1.16 log10, respectively, which further supports the use of MMV or PPV as a conservative surrogate for large viruses such as XMuLV by small virus filters (18,19). Infectious virus was detected in 48/105 MMV tests and 22/43 PPV tests. It is important to note that detection of infectious virus did not affect the robust and effective nature of the viral filtration step. PES filter mean clearance (5.87 ± 0.91 LRF) was higher than RC filter mean clearance (5.03 ± 1.17 LRF) at 95% confidence due to more frequent virus breakthrough as shown in Figure 6. Total virus load [log10 plaque-forming units (PFU)] and pH had statistically significant influences on parvovirus clearance, and there were differences between filter materials. High virus challenge increases parvovirus clearance for both PES and RC filters (P < 0.0001 and P = 0.04, respectively), but there is a higher rate of virus breakthrough when RC filters are challenged with greater than 0.6 log10 PFU/cm2, as shown in Figure 6. Frequency of virus passage is constant for PES filters over the range of 1.5–3.0 log10 PFU/cm2. Figure 5 shows that the highest PES filter LRF is reported at slightly acidic pH (P = 0.0019), but this sensitivity is not observed in RC filters (P = 0.20). The single data point representing <4 LRF clearance in PES filters was among the most basic operating conditions at pH 8.5, although pH effect remains significant if this point is removed from analysis.
Logistic fit of infectious parvovirus for PES (left) and RC (right) filters indicates RC filters are susceptible to virus breakthrough when challenged with load over 0.6 log10 per cm2. (○) indicates virus infectivity reduced below detection limits. (×) indicates infectious virus detected.
One-way analysis of parvovirus removal by PES filters (top) and RC filters (bottom) including the mean (black) and a fit line with 95% shaded confidence interval (gray). (○) indicates virus infectivity reduced below detection limits. (×) indicates infectious virus detected.
Recombinant protein type (IgG1, IgG4, other), pressure, mass loading (g/m2), volumetric loading (L/m2), and concentration (g/L) had no significant influence on reported clearance (P > 0.05).
Conclusion
Viral clearance data sharing between biotechnology firms has resulted in heterogeneous data sets for viral inactivation and viral filtration that are conducive to statistical review, facilitating scientific discussions for a risk-based approach to process characterization.
The shared data set shows pseudorabies (SuHV-1) is more readily inactivated under conditions that are effective for retrovirus. This observation may form the foundation for assessing XMuLV alone (the current scope of the ASTM standard E2888-12) as a sufficient model for low-pH inactivation. Reduced inactivation kinetics at 2–8 °C highlight the importance of product-specific characterization for recombinant proteins that cannot tolerate room temperature processing. Our data set supports ASTM standard E2888-12.
Small virus filters reliably reduce viral infectivity below assay detection limit for retroviruses, herpesviruses, and picornaviruses. Reovirus clearance was effective in all cases. This historical review provides further rationale for viral filtration validation studies with parvovirus models only, representing worst-case viral safety claims. For parvovirus validation, the database shows performance is insensitive to many process parameters, while viral clearance test artifacts such as virus challenge could have important effects on viral clearance.
The authors thank Justin Weaver (Alexion, Inc.), Tom Klimek (Eisai, Inc.), and Norbert Schuelke (Takeda Pharmaceuticals Co. Ltd.) for careful review of the manuscript.
Conflict of Interest Declaration
The authors declare there are no financial or nonfinancial competing interests related to this manuscript.
Footnotes
DISCLAIMER: The following article is a special editorial contribution from the BioPhorum Operations Group (BPOG). Please note that it did not go through the PDA Journal of Pharmaceutical Science and Technology regular peer review process.
- © PDA, Inc. 2016