Abstract
Terminal sterilization is considered the preferred means for the production of sterile drug products because it affords enhanced safety for the patient as the formulation is filled into its final container, sealed, and sterilized. Despite the obvious patient benefits, the use of terminal sterilization is artificially constrained by unreasonable expectations for the minimum time–temperature process to be used. The core misunderstanding with terminal sterilization is a fixation that destruction of a high concentration of a resistant biological indicator is required. The origin of this misconception is unclear, but it has resulted in sterilization conditions that are extremely harsh (15 min at 121 °C, of F0 >8 min), which limit the use of terminal sterilization to extremely heat-stable formulations. These articles outline the artificial nature of the process constraints and describe a scientifically sound means to expand the use of terminal sterilization by identifying the correct process goal—the destruction of the bioburden present in the container prior to sterilization. Recognition that the true intention is bioburden destruction in routine products allows for the use of reduced conditions (lower temperatures, shorter process dwell, or both) without added patient risk. By focusing attention on the correct process target, lower time–temperature conditions can be used to expand the use of terminal sterilization to products unable to withstand the harsh conditions that have been mistakenly applied. The first article provides the background, and describes the benefits to patient, producer, and regulator. The second article includes validation and operational advice that can be used in the implementation.
LAY ABSTRACT: Terminal sterilization is considered the preferred means for the production of sterile drug products because it affords enhanced safety for the patient as the formulation is filled into its final container, sealed, and sterilized. Despite the obvious patient benefits, the use of terminal sterilization is artificially constrained by unreasonable expectations for the minimum time–temperature process to be used. These articles outline the artificial nature of the process constraints and describe a scientifically sound means to expand the use of terminal sterilization by identifying the correct process goal—the destruction of the bioburden present in the container prior to sterilization. By focusing attention on the correct process target, lower time–temperature conditions can be used to expand the use of terminal sterilization to products unable to withstand the harsh conditions that have been mistakenly applied. The first article provides the background, and describes the benefits to patient, producer, and regulator. The article manuscript includes validation and operational advice that can be used in the implementation.
- Terminal sterilization
- Aseptic processing
- Sterilization
- Biological indicator
- Bioburden
- Probability of a non-sterile unit (PNSU)
- Regulation
- Sterility assurance
The first part of this series explained the production gap between aseptic processing (AP) and terminal sterilization (TS) that has existed in the pharmaceutical industry for many years. The operating gap is the result of a complete misinterpretation of what sterilization processes can actually accomplish and the subsequent application of inappropriate criteria by regulators and practitioners.
It is essential to understand that routine sterilization processes are intended to destroy the bioburden that microorganisms present on materials that will actually be used or administered to patients. It is that usage where the expectations for sterility apply. Because sterility cannot be confirmed by destructive means or testing, it is established through validation activities that support the efficacy of the sterilization process. In the validation exercise, biological indicators (BIs) are employed as worst-case surrogates for the bioburden. The use of BIs of greater resistance and higher population than the bioburden affords several advantages:
The BI is easy to use, recover, and test post-cycle.
The resistance of the BI is constant over time.
The BI can be placed within the item without altering item/container integrity.
Destruction of the BI provides enhanced confidence in destruction of the bioburden.
The BI reduces the need to determine bioburden population and resistance.
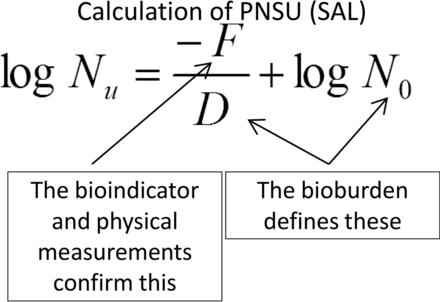
This last point is salient, as demonstration of the minimum probability of a non-sterile unit (PNSU) is the expected goal of all sterilization processes (1). Because the PNSU is an expectation of every cycle, its determination should be based upon the impact on the bioburden that is present in every cycle. [The BI is present only during the validation exercise, and while the BI population and resistance could be inserted into the PNSU calculation, doing so generally requires the use of sterilizing conditions that are excessive and well beyond those necessary for reliable destruction of the bioburden. These more aggressive conditions have a number of deleterious effects on the materials to be sterilized and should only be used where the materials being sterilized are minimally affected by their use. Examples of the negative impacts of excessive conditions on items being sterilized include one or more of the following: reduced potency of active pharmaceutical ingredient; increased degradation materials; increases in extractables/leachables; increase in particles, both visible and sub-visible; loss or weakening of package integrity; changes in physical appearance; changes in physical properties; reduced growth promotion (laboratory media); and other effects.] Thus the PNSU determination using the equation as shown in Figure 1 can be utilized by inserting known or estimated values for bioburden resistance and population.
Calculation of probability of a non-sterile unit, where: Nu = PNSU; D = D-value of the natural bioburden (calculated or assumed); F = F-value (lethality) of the process; N0 = bioburden population; Abbreviations: PNSU, probability of a non-sterile unit; SAL, sterility assurance level.
Applying the PNSU calculation to a low-temperature sterilization process requires three pieces of information: bioburden population, bioburden resistance, and a means for reporting process lethality.
Bioburden Population
The use of a low-temperature sterilization process is best accomplished following aseptic filling, as it provides the highest assurance of low population. A properly designed aseptic facility operated with the conventional controls on materials, equipment, personnel, procedures, and other elements is conventionally used to produce sterile products in which no microorganisms are present in the filled containers (2). The N0 for aseptic filling in the PNSU equation should be understood as 0 colony-forming units (cfu). A non-aseptic fill is certainly possible; however, the pre-sterilization bioburden in those instances must be as nearly as well controlled as for an aseptic fill. [The author has visited terminal sterilization facilities where aseptic filling is not performed (e.g., no media fills are performed); however, the other controls in these non-aseptic filling operations mimic those of aseptic processing, and pre-sterilization bioburden monitoring for population and resistance is performed in conjunction with the sterilization of each load.]
Bioburden Resistance
Before considering the resistance of the bioburden, some assumptions must be made relative to its identity.
The detected isolates from manned cleanrooms are mostly Gram-positive cocci.
The predominant isolates in manned cleanrooms are associated with personnel (3). These are primarily from the genera Staphylococcus and Micrococcus.
Sterilizing filtration of the solution and sterilization of the container-closure components will largely exclude spores from the filled containers.
The presence of any non-recoverable microorganisms as detectable by some of the new non-growth-based detection systems should not suggest patient risk because these microorganisms are not associated with human disease.
The vast majority of microorganisms associated with infectious disease lack meaningful resistance to moist heat even at temperatures well below 100 °C (4). (Bacillus cereus is associated with intestinal disorders and skin infections. Its presence in the pre-sterilization bioburden requires increased time at temperature than other infectious agents.)
Spore-forming microorganisms are unlikely to be present as spores in the cleanroom environment due to the availability of both humidity and sources of carbon, which favor the vegetative state of the microbial life cycle. The use of a sporicidal agent on a rotational basis serves to further restrict the presence of spores.
Process Lethality Estimation
Moist heat sterilization relies heavily on the General Method for determination of process lethality as originally developed by the food industry (5). The General Method provides for the use of periodic temperature measurement to approximate the lethality delivered over the full process duration. The General Method is calculated using eq 1:
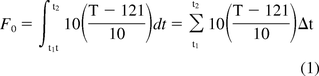 where:
where:
F0 = accumulated process lethality
t1 = process start time
t2 = process end time
T = Temperature
121 = assumed base temperature of 121 °C
10 = assumed z-value of 10 °C
Δt = time interval in minutes
The F0 determined by the General Method can be utilized in the PNSU equation to determine the safety afforded by the sterilization process (see Figure 1). The D-value for the non-spore-forming (vegetative) microorganisms in the bioburden is miniscule and is never used in practice (see Table I from Part 1 of this series).
Equivalent Lethality at Selected Time–Temperature Conditions
An identically configured equation (see eq 2) has been adopted for thermal decontamination of vegetative microorganisms in washers at elevated temperatures and is described in further detail in ISO 15883-1 (6). As a means for control of pathogenic microorganisms in hospital environments, the treatments supported have proven beneficial to patient welfare.
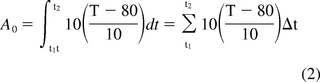 where:
where:
A0 = accumulated process lethality
t1 = process start time
t2 = process end time
T = Temperature
80 = assumed base temperature of 80 °C
10 = assumed z-value of 10 °C
Δt = time interval in seconds
This equation employs a base temperature of 80 °C and assumes a z of 10 °C. The process duration in the A0 equation is reported in seconds, suggesting a rapid destruction of vegetative cells at temperatures well above ambient. In using the A0 equation, the resistance of the microorganisms should be established at 80 °C.
The F0 and A0 equations are best suited for the evaluation of processes operating at close to the base temperature, as this provides the most accurate means for evaluating conditions close to the base condition. The z-values for microorganisms used in these equations should be those established at the base condition. When using moist heat sterilizing conditions at temperatures near 100 °C, the use of a base temperature closer to the operating condition would provide a more accurate means for evaluation of processes operating close to that temperature. The author proposed a similarly formatted equation with a 100 °C base condition some years previously, but it has received little application (7) (see eq 3). With the increasing application of moist heat at conditions near 100 °C it seems appropriate to suggest its adoption as a more appropriate means for measuring lethality and estimating the PNSU provided by these processes. For greater accuracy, the D- and z-values of the target microorganisms should be determined at 100 °C. This is easily accomplished by application of the boil test as described in USP <1229.2> Steam Sterilization of Aqueous Liquids (8).
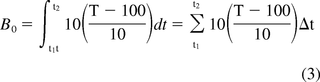 where:
where:
B0 = accumulated process lethality
t1 = process start time
t2 = process end time
T = Temperature
100 = assumed base temperature of 100 °C
10 = assumed z-value of 10 °C
Δt = time interval in minutes
A comparison of the lethality determination methods at different operating conditions supports the utility of B0 as a suggested means for use with processes operating near 100 °C (see Table I).
The lethality based upon the specified condition is used in conjunction with the D-value of the microorganism at the base temperature for best results. If the D-value is accurately known at the specified condition, the calculated PNSU will be the same regardless of the lethality method used.
Biological Indicator (BI) Choices
There are a number of well characterized commercially available BIs to use for processes operating near 100 °C. Bacillus subtilis 5230 ATCC 35021 is available in multiple formats and from various suppliers and is specifically intended for use with low-temperature moist heat process mostly at temperatures in the 110–118 °C range (9). For lower temperatures, B. atrophaeus ATCC 9372 or ATCC 49337, normally a BI intended for use with dry heat sterilization, can be an excellent choice (10). A recent publication supports the use of Bacillus oleronius for low-temperature moist heat sterilization processes (11). Other possibilities for BI selection are available. Spores of other Bacilli, or less desirably Clostridia, may possess appropriate resistance at temperatures closer to 80 °C. When low-temperature moist heat sterilization processes come into more common usage, other BI choices are likely to emerge. The use of the bioburden approach for sterilization provides another means for the validation of these processes (1). Using a worst-case isolate from the pre-sterilization bioburden might provide a direct means to validate these low-temperature processes.
Regardless of the microorganism chosen as the BI for these processes, it is essential that its resistance (D and z-values) in the formulation be determined at the sterilization conditions. Extrapolation from other conditions or substrates is ill advised.
Process Equipment
The equipment necessary to deliver these processes is already in widespread use. Steam-air-water sterilizers are in use for terminal processes above 100 °C, and they are easily adaptable to terminal sterilization processes operating in the 80–100 °C range. Alternatively, immersion sterilizer designs as used in the food industry could be employed for the lower temperature processes.
Conclusion
It should be evident from the content provided in this article and its Part 1 companion that the implementation of low-temperature moist heat sterilization needs only the will to execute it. The scientific justification and technical means for execution are already in place. The only restraints upon industry are self-imposed, arbitrary, and easily overcome.
Conflict of Interest Declaration
The author declares that he has no competing interests.
- © PDA, Inc. 2017