Abstract
The sensitivity of drugs to one or more elements of the primary packaging is a serious concern for the pharmaceutical industry. Biologics in particular are highly sensitive, leading to a higher risk of incompatibility and stability test failures as worst-case scenarios. This potential incompatibility—and the consequent formulation instability due to the interactions between the drug and the primary container surface—may have multiple causes: the intrinsic nature of the container surface, leachables coming from the materials used, substances coming from the production process, silicone oil droplets, or other particles. The Alba primary packaging platform was designed in order to have the same interface between the drug and the glass container surface on the different primary packaging containers to minimize the emergence of instabilities at later stages during the formulation development. The Alba containers are internally treated with an innovative cross-linked coating based on silicone oil lubricant, and the additional rubber components have been selected to minimize the possible differences between the container typologies. This paper shows in deep detail the subvisible particle release reduction and the comparability of the performances of different containers, obtained using such technology. To demonstrate this improvement, different analytical methods for particle measurement were used on bulk containers, Alba-treated ones and containers from a standard production (spray-on siliconization). Considering that Alba containers are conform to the standard compendial testing and the amount of particles released from Alba-coated syringes was comparable to the bulk syringes for the first two mildly stressful methods, it was decided to develop and apply a more challenging method, such as an autoclave treatment for 1 h at 121°C, to better highlight the performances of this innovative technology. The data obtained, under the most stressful conditions, show a substantial reduction in the released particle concentrations compared to a spray-on siliconized container, with comparable performances for all the containers included in the Alba platform. The latter could heavily reduce drug formulation development timing, facilitating the transition from one container to another.
LAY ABSTRACT: The sensitivity of drugs to one or more elements of the primary packaging is a serious concern for the pharmaceutical industry. Biologics in particular are highly sensitive, leading to a higher risk of incompatibility and stability test failures as worst-case scenarios. This potential incompatibility—and the consequent formulation instability due to the interactions between the drug and the primary container surface—may have multiple causes: the intrinsic nature of the container surface, leachables coming from the materials used, or substances coming from the production process, silicone oil droplets, or other particles. The Alba primary packaging platform was designed to solve these problems associated with the interaction between the drug and its primary packaging. This paper shows in deep detail and with robust data the subvisible particle release reduction obtained using such technology.
- Primary packaging
- Visible particles
- Protein aggregation
- Protein formulation
- Silicone oil
- Subvisible particles
Introduction
The development of new drugs requires thorough and extensive formulation studies, with the aim of obtaining a safe, highly pure and effective product (1⇓–3). The assessment of the formulation stability—considering that a drug shelf life has a lower limit of 18 months (4), while 24 or 36 months is the desired target—is a time-consuming step, and the formulation has to be re-adjusted and re-verified when there is a change in the primary packaging. In the case of parenteral drugs, different primary packaging containers (vials, cartridges, blow-fill-seal bottles, infusion bags, syringes, etc.) interact with the enclosed formulation in different manners (5, 6) because of different manufacturing processes (e.g., the presence of silicone for syringes and cartridges, the influence of the glass-forming process, etc.) (7, 8), different materials of make (e.g., glass versus plastic containers), additional components (like plungers/stoppers, needles, needle shields) (9⇓–11), and distinct shapes (12). During the formulation of a new drug, it is usual to have a transition from a generally non-siliconized container, the vial, which is most common container in the pre-clinical and in the first phases of the clinical trials, to a pre-filled syringe (PFS) or other pre-fillable containers for injection, which are usually internally siliconized. Thanks to their easy use, patient comfort, and home-use potential, higher dosing accuracy, and less overfill, PFSs are becoming a favorite choice for parenteral packaging, especially in the case of high-end drugs, with a projected market of 8.3 billion units in 2021 (13, 14). In case an incompatibility emerges due to the primary packaging changing at the clinical trial process, the reformulation of the drug could be necessary, increasing costs and lengthening the time to market.
The same drug/container interface should be present on the different primary packaging containers that are used throughout the formulation development to minimize the possibility that instabilities emerge at later stages. The Alba platform is an innovative solution devised to address this challenge and to minimize the risks. The new platform of products has been developed in order to have, for all the formats, the same premium and optimized container surface in contact with the drug thanks to a tailored innovative inner silicone coating (Figure 1). To minimize the difference between the container typologies, an accurate rubber component selection was performed (15).
Alba glass primary packaging platform.
Several studies have been carried out on the Alba platform to prove all its claimed properties. The study presented in this paper is focused on the subvisible particle (SbVP) release propensity of Alba containers.
The coating of the Alba products (more details on the Alba process are given in the Materials and Methods section) is based on silicone oil 360 Dow Corning Medical Fluid, polydimethylsiloxane (PDMS). Thanks to its physical and chemical characteristics, PDMS is commonly used as a lubricant in injection systems (e.g. PFSs) to ensure that the plungers glide evenly and effortlessly during injections (7, 16, 17).
PDMS guarantees high mechanical performance, and it has been safely used in primary parenteral packaging for decades; nonetheless it may present some criticalities. The fluid behavior of the silicone material itself and the possible interactions with some aggressive drug formulations can affect the lubricant layer status, causing the issue of silicone detaching from the inner surface of the primary packaging and leaching into the solution (18). Moreover, some protein formulations stored in siliconized containers may give rise to protein aggregates due to interactions with the lubricant (19, 20). This effect is significantly accelerated by the mechanical agitation stresses induced by the manufacturing and transportation processes (21, 22). Even though the silicone droplet-like particles leaching into the solution are not the only players in protein aggregation (11, 23), it is generally recognized that reducing their concentration in protein pharmaceutical formulations improves the final quality of the product (24). Indeed, novel approaches are being investigated to mitigate the problems emerging from silicone oil droplets in PFSs, such as cross-linked silicone coating treatment or the use of specific polymeric solutions (20, 21, 25).
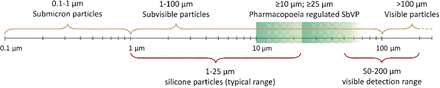
The European (26), American (27, 28) and Japanese (29) Pharmacopoeias provide upper limits regarding visible and SbVP concentration in parenteral solutions for various diameter ranges; the classification of different particle sizes is schematized in Figure 2. In particular, in the case of small-volume containers, the limits for SbVPs with dimensions ≥10 μm and ≥25 μm are set in USP <787> and in USP <789>—the latter in the case of ophthalmic solutions.
In this work, the amount of SbVPs released by the Alba coating under stressful conditions was investigated. In particular, two main objectives were pursued: the first was to develop a specific analytical particle methodology to verify and quantify the differences between the Alba coated syringes and the standard spray-on siliconized ones, using the bulk syringes as baseline; the second was to compare the various containers in the Alba platform. To reach the first objective, three experimental methods were used: (i) a method with testing conditions in compliance with USP <787> and <789> norms; (ii) a second method with longer agitation times; and (iii) a third method that puts the samples under severe physical stresses. Then, enforcing the most aggressive methodology, the stability of the coating across different batches and under accelerated aging conditions was investigated and, finally, the different containers included in the Alba platform were compared.
Materials and Methods
Materials
The following glass containers, provided by Nuova Ompi s.r.l. (Piombino Dese, Italy), were used for this study: 1 mL long luer cone washed and dried syringes (bulk glass, no lubricant), 1 mL long luer cone spray-on siliconized syringes, 1 mL long luer cone Alba syringes, 3 mL Alba cartridges, and 3 mL Alba vials. The bulk syringes were produced with for human use (FHU) materials, washed with water for injection (WFI), and dried in ISO 5 conditions without any lubricant addition. The spray-on siliconized syringes were produced with FHU material and processed in ISO 5 conditions, whereas the Alba containers were made of non-FHU material and processed under uncontrolled laboratory environment conditions; during the manual packaging phase, a laminar flow cabinet (Asalair 1200FLV, Asal s.r.l., Milan, Italy) was used to reduce the external particle contaminations. The standard syringes were spray-on siliconized with 0.5 mg/barrel Dow Corning 360 medical fluid 1000 centistokes (cSt); the Alba syringes, cartridges and vials were coated with Alba technology.
The Alba coating process is described in a pending patent (30); its main steps can be summarized as follows: the glass containers are preliminary washed with WFI and let dry, a silane coupling agent is then sprayed on the inner surfaces and a baking process is performed. The containers, after the just described pretreatment, are spray-on siliconized with Dow Corning 360 medical fluid 1000 centistokes (cSt), and the silicone is cross-linked with the use of an atmospheric plasma.
Particle Counting
The particle concentration analysis was performed using the Micro-Flow Imaging (MFI) 5200 series instrument (ProteinSimple, San Jose, CA, USA), driven by the MFI View Software—Version 2-R4.2.0.42.5211. The counting is performed by digital image analysis, and automatic particles identification is possible through the use of specific filters, which are helpful to discriminate between silicone oil, air bubbles, translucent particles, and so forth (31). The instrument is able to measure particle sizes that range from 1 to 70 μm, up to the maximum concentration of 900,000 particles per milliliter. In this study, for each container, the particle concentrations in the 1–70 μm size range were recorded.
Filling Solution Preparation
Distilled and 0.22 μm filtered water was used as filling solution (MilliQ water, Millipore, Milli Q IQ7000, Merck Millipore, Burlington, MA, USA). Different filling volumes were used depending on the containers capacity: the 1 mL long syringes were filled with 1.3 mL, while the 3 mL cartridges and the 3 mL vials were filled with 3.6 mL of MilliQ water. The filling volume for the syringes was chosen as the maximum volume that allowed the insertion of the plungers leaving 30 μL of air headspace. The values for the cartridges and vials, instead, represent 90% of their brimful capacity and was determined with the procedure described in the ISO 4802-2, par. 7.2: Determination of the filling volume (32).
Agitation Methods (Method I and Method II)
In Method I, the cone of each syringe was capped with an elastomer, FM30, from Datwyler Pharma Packaging (Alken, Belgium) and then the syringe was filled with 1.3 mL of water. To avoid liquid leakage during rotation, the filled containers were manually sealed using a vent tube, leaving roughly 30 μL of air as headspace; 1 mL Novapure 4023/50 ready-to-use grey plungers (West Pharmaceutical Services, Exton, PA, USA) were used. The syringes were placed on an automatic rotator and agitated at room temperature by an end-over-end rotation at 60 rpm for 20 times, as suggested by USP <787> and <789>. After having removed the plungers with metal tweezers, the solutions were slowly emptied over the back end of the syringes into particle-free plastic (PP) test tubes, which were immediately closed with their own caps. The tubes were then gently vortexed at low speed for 2 s and left to stand for 30 s to remove any air bubbles. Finally, 1 mL of solution was sampled from each PP tube with a micropipette and injected with an appropriate tip into the MFI instrument for measurement.
For Method II, the same procedures of in Method I were used, except for the agitation time that was prolonged to one week (7 days), maintaining the same 60 rpm rotation speed and room temperature conditions.
Autoclave Method (Method III)
The cone of each syringe was capped with a FM30 tip cap, from Datwyler Pharma Packaging, and then filled with 1.3 mL of water. To avoid liquid leakages or external contamination, each flange was covered with an aluminum foil, previously decontaminated by rinsing with acetone and water; the sample closing procedure described inside the European Pharmacopeia, paragraph 3.2.1, was used as a guideline (33). Samples were placed in an TL24 Autoclave (DeLama, San Martino Siccomario, Italy) in a vertical position for 1 h at 121 °C, the cycle followed the indications provided by the European Pharmacopeia (33). The temperature in the autoclave was monitored by a calibrated thermocouple. After the autoclave cycle, the samples were cooled down to room temperature. Following the same final procedures of Method I and II, the solutions were slowly transferred into PP test tubes, gently vortexed, and left to stand for 30 s. Then, 1 mL of solution was injected with an appropriate tip into the MFI instrument to perform the measurement.
The same procedures were also carried out for the other analyzed containers, except for the filling volume, which was, as previously described, 3.6 mL for the 3 mL cartridges and for the 3 mL vials, and for the closing method, which changed based on the container typology. The mouths of the cartridges were closed with lined caps 7236, 4780/7778 (West Pharmaceutical Services) and, after filling, the glaze areas were covered with aluminum foil. No rubber components were used to close the vial mouths; only the aluminum foil was applied after filling.
Results and Discussion
For the analytical method development, one of the most challenging containers in terms of silicone particle release and analyzable filling quantity was selected: the 1 mL long syringe. Its ratio between the inner siliconized surface area and the liquid filling volume is higher in comparison with the other Alba containers considered in this paper. In addition, the lower quantity of available solution implies an optimization of the sample preparation in the analytical methodology procedure.
After the development and identification of the most appropriate analytical approach using the 1 mL syringes, the other container formats were tested only with the final selected methodology, which resulted in being the autoclave method.
Results from Method I
The total particle release from the bulk, the standard spray-on siliconized, and Alba 1 mL long syringes was evaluated using the MFI instrument. The tests were performed on 30 samples for each category; the syringes were filled with 1.3 mL of distilled and filtered water, leaving a headspace of 30μL of air, and rotated end-over-end 20 times, as described in the Materials and Methods section. This kind of treatment allows the particulate matter to be properly mixed and suspended in the unit, inducing a mild stress on the samples.
During the end-over-end rotation the liquid was in contact not only with the glass container but also with the rubber component (plunger). To estimate the real particle contribution from the two different coating types, namely Alba and standard spray-on silicone, the bulk syringes were used as “baseline”.
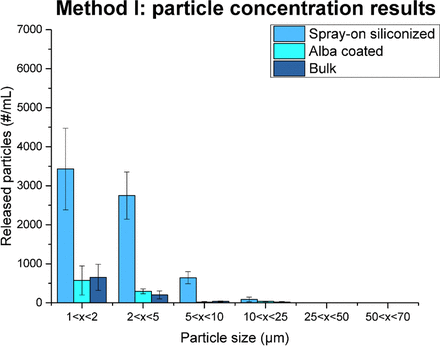
The test results for the three categories of syringes are displayed in Figure 3, in which the mean particle concentration is reported for different ranges of particle size and the error bars represent the standard deviation of the data. Compared to the spray-on siliconized syringes, the bulk and the Alba specimens released a lower amount of particles for all the analyzed size ranges, with almost an 80% reduction in the total number of particles. Considering that no significant difference was observed between the Alba and bulk syringes and that the glass containers for parenteral packaging can potentially be exposed to harsher conditions, both in terms of mechanical and heat stress, additional tests with more severe conditions were performed.
Particle release results from Method I. Bulk syringes, spray-on siliconized syringes (no fixation process), and syringes treated by Alba process results are reported for different particle sizes. The error bars in the graph represent the standard deviation of the data.
Results from Method II
To exert a harsher stress on the containers, additional methodologies were developed. Method II was conceived as a natural continuation of Method I, that is, the rotation time was prolonged to one week whereas all the other procedures were kept the same—see the Materials and Methods section. Particle release evaluation was performed by the MFI instrument over 30 samples for each category—that is, bulk versus spray-on siliconized versus Alba syringes. The results from Method II are reported in Figure 4.
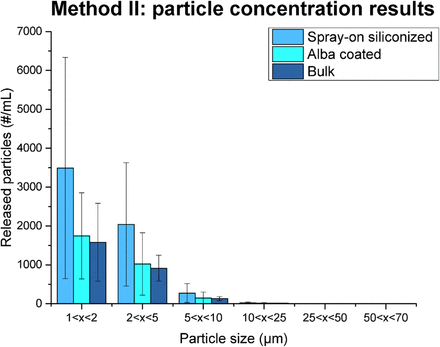
Particle release results from Method II. Bulk syringes, spray-on siliconized syringes (no fixation process), and syringes treated by Alba process results are reported for different particle sizes (40). The error bars in the graph represent the standard deviation of the data.
Also for Method II, the highest particle release was observed for the spray-on siliconized syringes and the overall number of particles was comparable between the bulk and Alba categories. With Method II, the total particle concentrations of all the three containers had the same order of magnitude of Method I, but an increase for both the bulk and Alba syringes was observed. For the bulk syringes, where the lubricant is absent, it is possible to suppose that this rise is not caused only by the glass itself but also by other variables, like a contribution from the rubber plungers. Surely, the same consideration is applicable to the other categories, but for the spray-on siliconized this could be less evident due to the higher values of particle concentrations and variability between samples. As a matter of fact, Method II significantly increased the standard deviations of the obtained data for all the categories.
Different adhesion forces between the glass and the two lubricant layers (Alba and spray-on silicone) could explain the different particle release propensities. For the spray-on siliconized syringes, the adhesion force between the silicone layer and the glass surface is characterized by a mild strength (7, 22, 25). For a certain degree of external solicitation, a fraction of particles from the silicone layer sloughs into the solution. Method I might be stressful enough to detach only a part of this fraction of silicone particles.
Given that the results obtained with the prolonged stress of Method II have the same order of magnitude of Method I and no significant difference was observed between Alba and bulk syringes, it is possible that these methodologies were not stressful enough to fully investigate the lubricant resistance.
Results from Method III
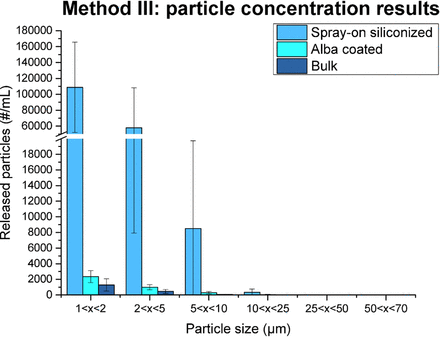
To better assess the Alba coating properties, Method III was developed—also termed the autoclave method in the following—which severely stresses the silicone layer. As discussed in the Materials and Methods section, samples subjected to Method III undergo a series of solicitations by different external agents, including temperature, pressure, liquid agitation, and mechanical stress. Figure 5 reports the results of Method III performed on 30 containers for each category.
Particle release results from Method III. Bulk syringes, spray-on siliconized syringes (no fixation process), and syringes treated by Alba process results are reported for different particle sizes. The error bars in the graph represent the standard deviation of the data.
From Figure 5, it is possible to notice that a higher concentration of particles was obtained with Method III and that the difference between the spray-on siliconized syringes and the bulk/Alba containers increased dramatically in comparison with the results obtained from Methods I and II.
By highlighting the disparity between the two coated categories, namely the Alba and spray-on siliconized categories, the autoclave method confirmed the trend emerging from the previous methodologies: Alba syringes performed better in terms of particle release than standard spray-on siliconized syringes, proving the increased adhesion strength between the silicone layer and the inner surface of the containers.
The spray-on siliconized syringes released a number of particles larger than the bulk and Alba samples, especially when comparing small particles. A difference of two orders of magnitude was observed between the spray-on siliconized syringes and the other categories for the 1 < × < 2 μm and 2 < × < 5 μm particles range concentrations. For larger particles, that is, in the 25 < × < 50 μm and 50 < × < 70 μm ranges, the concentrations were similar to the ones obtained by the other two analytical methods.
Another interesting result is that after the autoclave treatment the bulk containers released lower amounts of particles compared to Method II. This corroborates the previous hypothesis of particle contamination from the rubber plunger components during the one week rotation, as no rubber component was used in Method III.
Method III was the only one that showed a significant difference between Alba and bulk syringes and, therefore, was considered the most appropriate methodology to investigate the stability of the new coating across different batches, under accelerated storage conditions, and between different container types.
Stability of the Alba Coating across Different Batches
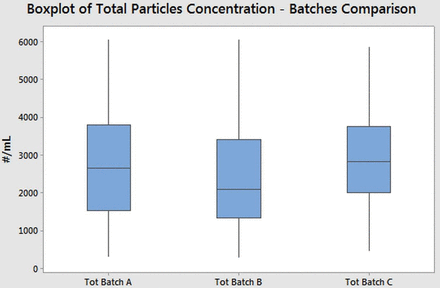
After the superior results obtained comparing Alba and spray-on siliconized syringes, the repeatability of the Alba process was investigated. An ISO 2859-1:1999(E) (34) sampling approach was used on three different batches of syringes, each with a number of produced items in the range of 501–1200 units. Eighty syringes for each batch were analyzed with the autoclave method, applying the general inspection level two and a “J” sample size.
The results are illustrated in Figure 6, in which the summation of the concentration of particles between 1 μm and 70 μm is shown. The total particle concentration and its variability were comparable between the three different batches. Considering that the statistical populations were not normal, an analysis of variance (ANOVA) hypothesis test was not applicable. Instead, the comparability of the batches variances was verified with Levene's test (P-value > 0.05).
Boxplot of total particle (dimensions between 1 μm and 70 μm) concentrations (obtained with the MFI instrument) from three different Alba syringe batches. Each batch consisted of 80 syringes, and the samples were tested applying Method III guidelines.
Stability of the Alba Coating under Accelerated Storage Conditions
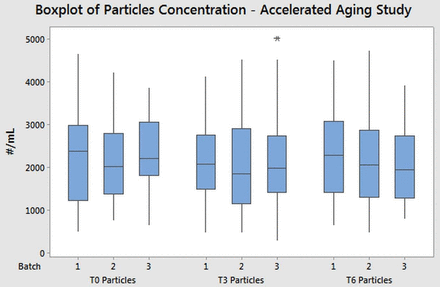
In order to verify the stability and the shelf life of the Alba containers, an accelerated aging study was performed on Alba syringes. The ICH guideline (35), Southern African Development Community (SADC) guideline for stability testing (36), and the ASTM F1980-07(2011) standard (37) were followed as reference conditions for the study (see Table I). Three different batches of syringes were stored empty and in a vertical position (flange up) at 40 ± 2 °C and 75 ± 5% relative humidity (RH) for 6 months inside a certified climatic chamber (Table I).
ICH Storage Condition Guidelines (35)
Thirty samples from each of the three batches were analyzed with the autoclave method at three different time points: immediately after production, after 3 months, and after 6 months of storage. In Figure 7, the results are expressed as summation of the concentrations of all the particles between 1 μm and 70 μm.
Boxplot of total particle (dimensions between 1 μm and 70 μm) concentrations (obtained with the MFI instrument) from the Alba aging study. Each batch was composed of 30 syringes, and the samples were tested applying Method III procedures.
The results showed comparable total particle concentrations over time for all the three different batches. As the data satisfied the normality hypothesis, an ANOVA test was performed on the particle summation for each time point. The analysis confirmed the comparability between the three mean values (P-value > 0.05). The three populations showed normal data distribution and variance equivalence (Levene's test, P-value > 0.05).
Efficacy of the Alba Coating across Different Container Types
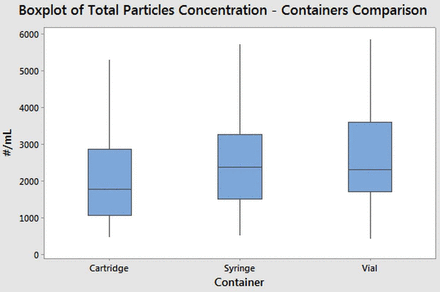
In order to compare the particle release of the different types of containers in the Alba platform, the autoclave method was applied on Alba 1 mL long syringes, Alba 3 mL cartridges, and Alba 3 mL vials. For each category, 60 containers were tested.
The experimental data (illustrated in Figure 8) demonstrates that the particle concentrations from all the three Alba coated containers are comparable. These results prove that it is possible to apply the Alba coating process on different container shapes, obtaining comparable performances with respect to the particle release propensity.
Total particle (dimensions between 1 μm and 70 μm) concentrations (obtained with the MFI instrument) comparing the different Alba containers, 60 samples for each category. The samples were tested applying Method III guidelines.
Conclusions
In this study, the release of SbVPs from bulk syringes, spray-on siliconized syringes, and syringes coated with an innovative process (Alba) were compared using three different testing methods.
Results from the least aggressive methodology showed a 4-fold reduction in particle concentration for Alba and bulk syringes compared with spray-on siliconized containers. When the filled samples were rotated for one week, Alba syringes still performed similarly to the bulk syringes, even if the difference with the spray-on silicone category decreased.
In order to significantly challenge the new coating process and assess its potential, an autoclave treatment (1 h at 121 °C) was developed and applied; it exerted a more severe stress on the specimens. Remarkably, the number of released particles from the Alba samples was almost 2 orders of magnitude lower than the standard spray-on siliconized syringes and double in comparison with the bulk category.
To demonstrate the repeatability of the Alba process, three different production batches of coated syringes were analyzed with the most stressful method, showing comparable results. To verify the Alba coating stability, the syringes were incubated for 6 months at 40 °C 75% RH and analyzed, showing comparable particles results during the whole incubation period.
All the containers belonging to the Alba platform, that is, syringes, cartridges, and vials, showed comparable particle concentrations, demonstrating that the same drug/container interface was exposed to the drug. This could heavily reduce the drug formulation development timings, facilitating the transition from a container to another.
Additional characterizations of the Alba platform, in terms of functional performances and chemical stability, will be covered in future publications.
Conflict of Interest Declaration
The authors herewith declare they do not have any financial or non-financial competing interests related to the content of the article.
- © PDA, Inc. 2018