Abstract
The process capability and potential for various forms of chromatography to remove viruses have been discussed extensively in the literature, including the observed variability in performance of some unit operations such as Protein A and cation exchange (CEX). Some unit operations such as anion exchange (AEX) have shown robustness over a wide range of operating conditions. The robustness and effectiveness of the AEX step combined with a detailed understanding of the mechanisms that result in virus and impurity partitioning versus protein partitioning (e.g. conductivity, loading, pH range) support the feasibility of a generic or modular claim for AEX. A more fundamental understanding of the mechanisms for other chromatographic media (Protein A, CEX, and mixed-mode) could lead to more effective and more robust log reduction value (LRV) claims for these steps as well. Specific examples of CEX and mixed-mode chromatography were explored in the session and were also discussed in detail at the 2013 and 2015 Viral Clearance Symposia. Although some gaps remain in the mechanistic understanding of these unit operations, significant progress has been made and was reported at the 2017 Viral Clearance Symposium. It is important to note that recent publications on the mechanisms of viral clearance for mixed-mode chromatography and a framework for measurement of relative hydrophobicity have provided insights and new tools to better define the operating space and critical process parameters. The session also explored the use of next-generation mixed-mode adsorbers and the potential mechanisms contributing to the observed viral clearance. Gaps were also identified (e.g. integrity test when size-based mechanisms are used) and should be addressed to ensure robust viral clearance for these integrated and productive emerging unit operations.
LAY ABSTRACT: Preparative chromatography is the most widely used unit operation for purification of therapeutic proteins. This session focused on the potential for various forms of chromatography to remove viruses. To advance to the next level of process, understanding the virus removal mechanism of different types of chromatography was addressed.
- Viral clearance
- Downstream processing
- Affinity chromatography
- Ion exchange chromatography
- Multimodal chromatography
- Membrane adsorbers
Introduction
The process capability and potential for various forms of chromatography to remove viruses have been discussed extensively in the literature, including the observed variability in performance of some unit operations, such as Protein A and cation exchange (CEX) (1⇓–3). Some unit operations, such as anion exchange (AEX), have shown robustness over a wide range of operating conditions (2, 4). The robustness and effectiveness of the AEX step combined with a detailed understanding of the mechanisms that result in virus and impurity partitioning versus protein partitioning (e.g., conductivity, loading, pH range) support the feasibility of a generic or modular claim for AEX.
A more fundamental understanding of the mechanisms for other chromatographic media (Protein A, CEX, and mixed-mode) could lead to more effective and more robust log reduction value (LRV) claims for these steps as well.
Specific examples of CEX and mixed-mode chromatography were explored in the session and were also discussed in detail at the 2013 and 2015 Viral Clearance Symposia (5⇓–7). Although some gaps remain in the mechanistic understanding of these unit operations, significant progress has been made and was reported at the 2017 Viral Clearance Symposium. It is important to note that recent publications on the mechanisms of viral clearance for mixed-mode chromatography (8) and a framework for measurement of relative hydrophobicity (9) have provided insights and new tools to better define the operating space and critical process parameters. The session also explored the use of next-generation mixed-mode adsorbers and the potential mechanisms contributing to the observed viral clearance. Gaps were also identified (e.g., integrity test when size-based mechanisms are used) and should be addressed to ensure robust viral clearance for these integrated and productive emerging unit operations.
2.2 Case Studies Reviewed at the Conference
2.2.1 Viral Clearance Potential of Chromatography Processes
Kang Cai and Matthew Dickson, MedImmune:
A summary of >60 viral clearance studies for Protein A, AEX operated in flow-through mode (AEX F/T), and CEX was presented. The analysis included both monoclonal antibody (mAb) and fusion proteins, and the range of viruses explored included xenotropic murine leukemia virus (XMuLV), pseudorabies virus (PRV), minute virus of mouse (MVM), porcine parvovirus (PPV), reovirus type 3 (Reo3), and simian virus 40 (SV40) with results summarized by virus class.
Protein A had variable LRV ranging from 1 to 4 with similar clearance for XMuLV and parvovirus. AEX operated in a flow-through mode at conditions within the previously described bracketed operating space (2). Specifically, robust clearance was achieved with XMuLV over a wide range of pH operating conditions (5.3–8.2) and conductivity <11 mS/cm. Parvovirus removal was more variable (range, 1.5–7.8 LRV) and low (<2 LRV) when operating under pH conditions near or below the isoelectric point (pI) of the virus. The conclusion from these observations was that the difference in LRV for XMuLV was attributed to tighter binding than parvovirus even though the pI of the two viruses differs only slightly (XMuLV pI = 5.8; parvovirus pI = 6.2).
The observations for CEX chromatography were that LRV for XMuLV was variable, while viral clearance for parvovirus was consistently low. A further exploration on the impact of loading and elution conditions for 24 specific studies revealed that when elution was performed in the pH range of 5.0 to 6.1 combined with moderate salt (11 studies) elution, the LRV for XMuLV was on average 4.29 with robust performance. LRV was slightly increased for XMuLV when a wash step was added. Increasing the elution pH above 5.9 or using high salt elution conditions resulted in poor XMuLV viral clearance (LRV ≤2). The results for XMuLV clearance as a function of pH and the observation of robust clearance for a pH ∼5 are consistent with previous published results of Connell-Crowley et al. (10). Connell-Crowley et al. also reported low LRV for Mouse Minute Virus (MMV) (LRV generally <2) and good clearance for PRV (LRV > 4.5). Cai and Dickson, the authors of the presentation at the Symposium, postulated that under high pH elution conditions for CEX (average pH 7.3), higher clearance for PRV was related to the higher pI (7.1) of this virus versus the other viruses evaluated (XMuLV pI = 5.8; parvovirus pI = 6.0–6.2; Reo3 pI = 3.8; SV40 pI = 5.4).
2.2.2 Non-Platform AEX Viral Clearance
Richard Chen, Eli Lilly:
As part of an antibody purification platform where 17 mAbs were evaluated, AEX F/T has been shown to be a robust viral clearance unit operation for multiple molecules (early, late, and commercial). However, for two particular molecules, mAb C and mAb D, AEX did not provide robust viral clearance (LRV < 4; XMuLV range from 2.84 to 3.43; MMV < 1). Two case studies were presented summarizing the reasons behind these outliers, the adjustments required to ensure adequate overall viral safety, and future considerations for subsequent development.
The first case study involving mAb C had a pI of 8.1 to 8.6 and exhibited weak binding on AEX at neutral processing pH. An AEX process was developed at pH 6.2 and higher conductivity (12 mS/cm) but achieved lower-than-expected LRV (XMuLV range from 2.84 to 3.43; MMV <1). The hypothesis is that configuration of mAb causes it to act as a zwitterion (i.e., net negative charge at neutral pH). It is important to note that these operating conditions were at substantially lower pH than had previously been shown to achieve robust viral clearance (2).
The second case study was mAb D with a pI range from 7.5 to 7.9, which is more acidic/neutral than other mAbs, and it exhibited weak binding on AEX at neutral processing pH's resulting in low yield. An AEX step was developed using citrate pH 7.4 (∼5 mS/cm) with lower-than-expected viral clearance LRV observed (XMuLV range from 2.30 to 2.52; MMV range from <1 to 1.35).
2.2.3 Virus Clearance Data for Mixed-Mode Resins
Holger Hoffmann, Sanofi:
Multimodal or mixed-mode chromatography (MMC) is becoming increasingly popular in antibody purification owing its unique selectivity. The resins are salt-tolerant and could adsorb target proteins with the combination of ionic interactions, hydrogen bonds, and hydrophobic interactions. Because of these characteristics, mixed-mode resins display high product- and process-related impurity removal capacity and can alternatively be used for a direct capture of the target protein from the harvested cell culture fluid. However, limited virus clearance data for mixed-mode resins are available.
Virus clearance data for Capto Adhere in bind and elute mode with different monoclonal antibodies showed clearance of the retrovirus MuLV with values of ∼5 log10 for all three mAbs. The virus clearance for the parvovirus MVM is only contributive with <3 log10. Capto MMC in bind and elute shows only poor virus clearance with values <2 log10. The virus clearance for Capto Adhere in the flow-through mode was investigated with three mAbs as a function of buffer pH. The clearance for MuLV was >5 log10 between pH 5.8 and pH 7.3 and dropped to 4 log10 at pH 4.4. The clearance values for MVM showed a similar trend with the highest LRV of 7 log10 at pH 7.3 and ∼4 log10 between pH 4.4 and pH 5.8.
Overall, Capto Adhere in the flow-through mode showed robust virus clearance for MuLV and MVM over a broad pH range from 4.4 to 7.3. In the bind and elute mode, however, only contributive properties for MVM were determined. Furthermore, it seems that Capto Adhere is superior to Capto MMC for the bind and elute operation. Poor virus clearance results were obtained with Capto MMC for both viruses.
2.2.4 Taking Advantage of Simple Adsorptive Depth Filtration to Substitute Anion Exchangers for Virus Clearance in Monoclonal Antibody Processes
Alexander Faude, Rentschler
Introduction
AEX (or AIEX) chromatography is a commonly used process step to show the potential for virus clearance. Because mAb platform processes are improved continuously, the AIEX is often not necessary in the DSP regarding product purity. Therefore, the AIEX is still used for virus safety only as an orthogonal virus removal step to virus filtration, inactivation, and ProA chromatography. Adsorptive depth filtration (DeF) is a unit operation in most mAb processes and has the potential to substitute the AIEX for virus removal.
Depth Filter Types Tested for Virus Removal
The following two different types of depth filters were evaluated for their virus removal potential: a conventional depth filter with positively charged resin and diatomaceous earth in two layers with different porosity—Zeta Plus 90ZB05A, 3M (porosity: 3 μm 30ZB, 0.1 μm 90ZB)—and a synthetic depth filter with nonwoven highly Q-functionalized polypropylene strings—Emphaze AEX HP, 3M.
The depth filter properties can have virus retentive effects via multiple mechanisms including (1) electrostatic adsorption (substitution of AIEX chromatography), (2) size exclusion (orthogonality to virus filtration compromised), and (3) hydrophobic interaction (orthogonality to hydrophobic interaction chromatography/MMC compromised).
Virus Removal Results for Depth Filters
Removal of MuLV
Both filter types were evaluated under different conditions. The basis matrix was a generic buffer system containing 20 mM citric acid, 20 mM phosphoric acid, and 20 mM Tris plus NaOH for pH adjustment.
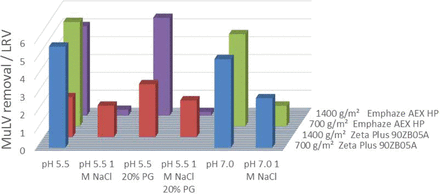
At a filter load of 700 gmAb m−2, the Zeta Plus 90ZB05A showed an LRV of >6 at pH 5.5 and pH 7.0 (Figure 1). Shielding of ionic interactions by 1 M NaCl at pH 7.0 resulted in an LRV of 3; therefore, an LRV of 3 can be claimed by ionic adsorption. At 1400 gmAb m−2 filter load, at pH 5.5, the LRV was 2.5. Shielding ionic interactions with 1 M NaCl LRV dropped to LRV 2, which means no significant virus removal by ionic adsorption was found. Suppressing hydrophobic interactions with 20% (w/v) propylene glycol, an LRV of 3 was found, which is comparable to the basis matrix. This can be interpreted as a negligible contribution of hydrophobic interactions to virus removal. The remaining LRV under suppressed ionic and/or hydrophobic interactions can be explained by size exclusion effects.
Results for murine leukemia virus (MuLV) removal by depth filtration with Zeta Plus and Emphaze AEX HP under different conditions.
The same conditions were tested for the Emphaze AEX HP. At both filter load densities, an LRV of >5 was observed at pH 5.5 and pH 7.0. Shielding of ionic adsorption by 1 M NaCl reduced the LRV to <1. Suppressing hydrophobic interactions with 20% (w/v) propylene glycol did not affect the high virus removal (Figure 1). Therefore, the LRV can be claimed completely by anionic adsorption.
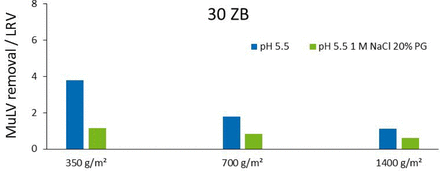
For investigation of the observed size exclusion effects, the conventional depth filter layer 30ZB with 3-μm pores was tested (Figure 2). An LRV of ∼4 was found in the base matrix with 200 gmAb m−2 filter load and no significant LRV with shielded ionic interactions. Therefore, virus removal can be claimed by ionic adsorption, as size exclusion effects are negligible with 3-μm pores and a virus size of 0.1 μm. The LRV of the 30ZB decreased strongly to an LRV of 2 at 700 gmAb m−2, and there was no significant LRV at 1400 gmAb m−2 filter load. This can be a result of less available filter surface caused by the higher porosity, resulting in overloaded conditions.
Results for MuLV removal by depth filtration with Zeta Plus 30ZB under different conditions.
Removal of MVM
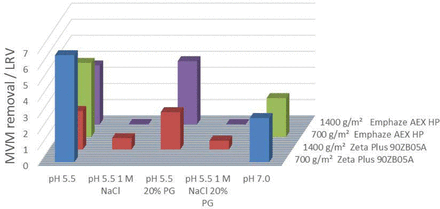
For MVM, the size exclusion effect was neglected based on filter porosities of 0.1 μm and a virus size of 20 nm. With 700 gmAb m−2 filter load, the Zeta Plus 90ZB05A showed an LRV of 7 at pH 5.5 and an LRV of 3 at pH 7.0 (Figure 3). At 1400 gmAb m−2 filter load, the LRV dropped to 3. Shielding of ionic interaction resulted in no significant virus removal by the step. Suppression of hydrophobic interaction did not affect the LRV. Therefore, the LRV can be claimed by ionic adsorption.
Results for MVM removal by depth filtration with Zeta Plus and Emphaze AEX HP under different conditions.
The Emphaze AEX HP also showed an LRV of 5–6 at pH 5.5 and less and an LRV of 3 at pH 7.0, again not affected significantly by the filter load density. As well as for MuLV, the LRV can be claimed by anionic adsorption, proven by the suppression experiments. Size exclusion and hydrophobic interactions are negligible.
Exemplary Results for Anionic Virus Retention
Table I provides a summary of LRV examples for anion exchangers and depth filters.
Examples of Virus Removal by Anionic Retention
For the classic quaternary anion exchange adsorbers Capto Q, Q Sepharose FF, and Sartobind Q, an LRV of 4 to >6 were achieved for MuLV in phosphate, citrate, and acetate buffers in the range of from pH 5.0 to 7.5. Removal of MVM was in the range from an LRV of 1.7 to 3.0 in phosphate and acetate buffers, respectively, whereby PPV removal was higher with an LRV of about 6. In citrate buffers, MVM removal was not significant in the range from pH 5.0 to 7.2. The anionic mixed-mode adsorber Capto Adhere showed an LRV of >6 for MuLV and an LRV of 2.3 for MVM, even in citrate buffer at pH 5.5.
LRVs for depth filters are shown in citrate buffer at pH 5.5. The Zeta Plus 90ZB05A showed an LRV of about 3, an impact of filter loading density on virus removal, even more than the 30ZB layer. The MVM removal was higher (LRV = 6.7) caused by the negligible size exclusion effect, but it also decreased with an increase in the filter load density.
The Emphaze AEX HP depth filter showed effective removal of MuLV and MVM in citrate buffer at pH 5.5 and was not impacted by filter loading density in the tested range.
Summary
Depth filters
For the Zeta Plus 90ZB05A, the LRV depends on protein load, while the size exclusion effects were only significant for only MuLV. For the Zeta Plus 30ZB layer, the LRV depends strongly on protein load, but no significant size exclusion effects were shown. For the Emphaze AEX HP, LRV was robust regarding protein load, and the virus retention was proven to be based on ionic interactions.
Depth Filters for Substitution of AIEX Chromatography
For the tested depth filters, anionic retention can be shown for a clear virus safety claim.
Conventional depth filters show limited binding capacities, which can be explained by competitive binding between protein and virus. The study was carried out with two different filter lots, thereby eliminating lot-to-lot variability.
Depth filters tolerate polyvalent anions like citrate even more than anionic mixed-mode adsorbers like Capto Adhere, whereby Q-resins or Q-membranes showed no citrate tolerance regarding MVM retention. For conventional depth filters, the citrate-tolerant interaction could be based on diatomaceous earth or the cationic resin. Emphaze AEX HP is Q-functionalized, and so far, the citrate tolerance mechanism is not understood.
2.2.5 Virus Clearance Capacity of an Anion Exchange Membrane Adsorber
Eileen Wilson, Michael McGarrah, Melissa Schofield, and Hiren Ardeshna, GSK:
Both AEX chromatography and AEX membranes are commonly used in mAb purification processes and these often provide robust virus clearance. Emerging AEX membrane adsorber technologies, such as the 3M Emphaze ST AEX Adsorber, have been designed to combine the benefits of chromatography (high ligand density) and membranes (faster processing, single use), as well as salt tolerance. The Emphaze ST Adsorber is a single-use device consisting of two layers, namely, a Q-functionalized nonwoven fiber and 0.8-μm polyamide guanidinium functionalized, salt-tolerant membrane. Acceptable product quality and process performance have been shown for use in a GSK mAb process in the flow-through mode for multiple mAbs (11).
In this experiment, the virus clearance capacity of the Emphaze ST Adsorber has been evaluated for a retrovirus and a parvovirus in an mAb [Chinese hamster ovary (CHO) cell culture–derived IgG1] purification process. For the virus-spiked experiments, four Q-functionalized layers and three guanidinium functionalized layers, kindly provided by 3M, were assembled in a filter holder, and the assembled device was equilibrated. The load material was Protein A eluate (host cell protein [HCP], 75.9 ng/mg; DNA, 7.7 pg/mg), which was titrated for low pH virus inactivation (VI), then adjusted to pH 7.5 and conductivity 7.5 mS/cm. The adjusted load was spiked with either MuLV or PPV at a high or low spike ratio per virus. The spiked load material was filtered per contract laboratory procedure and loaded on the Emphaze ST Adsorber at room temperature by a peristaltic pump at a constant flow rate. Pressure was monitored to not exceed the limit of 30 psi. Operating conditions are summarized in Table II. Virus concentration in load and flow-through measured by infectivity assay (TCID50) is shown in Table III. Results by qPCR (not shown) were similar, with no detectable virus in the flow through for either MuLV experiment or PPV Spike 1 and detectable virus in the flow through with the higher PPV challenge in Spike 2.
Emphaze ST Adsorber Virus-Spiked Experiments
Emphaze ST Adsorber MuLV and PPV Clearance by TCID50
The Emphaze ST Adsorber provided promising MuLV and PPV clearance for low pH-treated and neutralized Protein A chromatography eluate. The Emphaze ST Adsorber may replace both post-VI neutralization filtration (depth and membrane) and AEX F/T chromatography and possibly prefiltration for small virus retentive filters (VFs), thus enabling coupling of the VI-AEX-VF steps. Future work may include optimizing virus spike ratios to increase clearance (MuLV) and to limit breakthrough (PPV); evaluating a larger panel of model viruses; evaluating the impact of virus stock purity on performance and virus clearance; and identifying critical process parameters for virus retention (e.g., pH, conductivity, residence time, load ratio, load purity).
2.2.6 Evaluation of Synthetic Multi-Mechanism Clarification Device as a Virus Removal Step
Jena Daya, Regeneron:
Regeneron assessed a synthetic multi-mechanism clarification device for removal of XMuLV and MVM in monoclonal antibody manufacturing processes. The multimechanism clarification device is a single-use technology capable of substantially reducing viral load without the leaching associated with cellulose-based depth filters and the validation of resin lifetime associated with reusable chromatography columns. Because the device includes anion exchange and size-exclusion mechanisms, clearance of XMuLV and MVM were evaluated in separate prospective D-optimal multivariate experiments to provide evidence of a primary mechanism and to illustrate sensitivity to input parameters.
An XMuLV study (N = 11) was designed to evaluate the main effect of flow rate, while both main effects and quadratics were estimated for monoclonal antibody pI, pH and sodium chloride concentration of protein solution, and membrane loading (L/m2). A smaller MVM study (N = 6) was performed to evaluate the main effects of pH and sodium chloride concentration of protein solution. The device was operated in the flow-through mode such that the product passed through while the virus was retained. After processing the load material, a 2.0 M sodium chloride wash solution was passed through the device to disrupt electrostatic interactions, while size-based retention would putatively be unaffected.
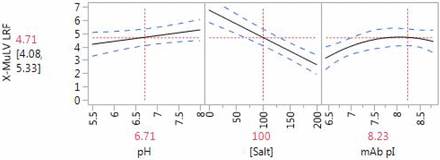
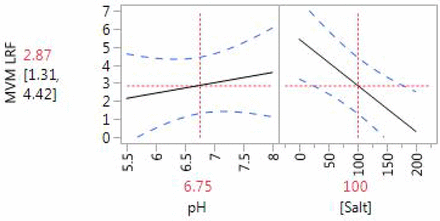
The synthetic multi-mechanism clarification device provided up to 7 log10 reduction factor for both viruses. Efficient recovery of both XMuLV and MVM in the 2.0 M sodium chloride fraction provided evidence that the primary mechanism is anion exchange. Least squares linear regression was performed and nonsignificant terms (p < 0.10) were removed, resulting in a linear model of XMuLV clearance as a function of pH, sodium chloride concentration, and pI. Both factors studied for MVM (pH and sodium chloride) were significant. The models for XMuLV and MVM are illustrated in Figures 4 and 5, respectively, with 95% confidence interval in light blue. The leverage of ionic strength and pH further supports the hypothesis that the primary mechanism for virus removal by the multi-mechanism device is electrostatic, while the significance of monoclonal antibody pI suggests that the product may compete with virus for binding sites. An electrostatic mechanism suggests the integrity of the single-use device as a virus removal step may be supported by methods similar to AEX membrane chromatography.
Predicted XMuLV log10 reduction factor for synthetic multi-mechanism clarification device.
Predicted MVM log10 reduction factor for synthetic multi-mechanism clarification device.
Conclusions and Future Work
The results presented in the session suggest that although there has been progress in characterizing viral clearance for a wide range of chromatography steps, some gaps still exist in the mechanistic understanding and the impact of process parameters on CEX. Robust clearance for multiple viruses was achieved when CEX was operated within a narrow parameter space (pH ∼5, low salt elution), which suggests that modulating the affinity of the viruses to the CEX via electrostatic interactions is key to achieving good viral clearance—an observation consistent with data previously presented in the literature. The relative difference between the virus pI and the operating pH in CEX may also play a role in viral clearance. However, additional experiments with a range of mAb and operating conditions will be required to confirm this hypothesis of mechanism. A combination of the results presented at the symposium and the literature suggests that these observations are likely independent of resin, as multiple resins were evaluated. Discussion at the Symposium focused on the concept of how the difference in pI of mAb and virus can affect the LRV for CEX based on the hypothesis that CEX is primarily a charged-based separation, analogous to AEX clearance.
The results from the combination of non-platform mAb/AEX conditions suggest that for operating conditions outside of the modular AEX space (2), where mAb partitioning is observed in the F/T mode, buffer composition may play a significant role in reduced viral clearance. Additional experiments with a range of mAb and buffer compositions are required to verify this hypothesis. One alternative to address this challenge could be the use of next-generation AEX resins, including salt-tolerant ligands.
A case study of the application of mixed-mode anion (MMAEX) and mixed-mode cation-exchangers (MMCEX) showed the feasibility of obtaining robust viral clearance for XMuLV for MMAEX in both F/T and bind and elute (B/E) modes, extending observations previously reported for the F/T mode (12). Clearance of MVM was robust in MMAEX F/T, while it was more limited in the B/E mode. Overall viral clearance for MMCEX was limited for the single resin evaluated. Similar to CEX chromatography, a more thorough investigation of the mechanisms and the impact of operating parameters may be required to achieve robust clearance for MMCEX.
Mixed-mode membrane adsorbers appear to have the potential to achieve an LRV of >4, but it can operate via multiple mechanisms (size exclusion, ionic, and hydrophobic). Hence, the mechanism of virus removal needs to be shown to define worst-case conditions for studies to evaluate the robustness of virus removal and to ultimately substantiate potential orthogonality claims to other unit operations including AEX.
Several gaps were identified, including the potential need for an integrity test for mixed-mode membrane adsorbers if the viral removal mechanism is size-based, and further characterization of the parameters that could impact viral clearance mechanisms, including pore size distribution, number/density/distribution of IEX or hydrophobic interaction chromatography binding spots, and the robustness of viral clearance versus pI of potential contaminants versus model viruses used in studies, similar to other chromatography systems.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2018