Abstract
In many downstream processes, chromatographic purification steps contribute significantly to the overall virus reduction capacity. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guideline Q5A(R1) outlines that “Over time and after repeated use, the ability of chromatography columns and other devices used in the purification scheme to clear virus may vary. Some estimate of the stability of the viral clearance after several uses may provide support for repeated use of such columns.” Virus reduction studies with used resin normally require a high amount of in-process material and time. The experience with used resin has been accumulated continuously, stimulating the discussion on whether it would be necessary to investigate virus reduction on used resins for each product. In this session, additional experience from studies with used resins was provided.
LAY ABSTRACT: In many downstream processes, chromatographic purification steps contribute significantly to the overall virus reduction capacity. An estimate of the stability of the viral clearance after several uses may provide support for repeated use of such columns. Virus reduction studies with used resin normally require a high amount of in-process material and time. The experience with used resin has been accumulated continuously, stimulating the discussion on whether it would be necessary to investigate virus reduction on used resins for each product. In this session, additional experience from studies with used resins was provided.
Background
In many downstream processes, chromatographic purification steps contribute significantly to the overall virus reduction capacity. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guideline Q5A(R1) (1) outlines that, “Over time and after repeated use, the ability of chromatography columns and other devices used in the purification scheme to clear virus may vary. Some estimate of the stability of the viral clearance after several uses may provide support for repeated use of such columns.” Virus reduction studies with used resin normally require a high amount of in-process material and time. The experience with used resin has been accumulated continuously, stimulating the discussion on whether it would be necessary to investigate virus reduction on used resins for each product. In this session, additional experience from studies with used resins was provided.
Evaluation of Performance Attributes Supporting Maintained Viral Clearance By Chromatography Columns At End of Lifetime
John Mattila, Regeneron
Regeneron has summarized virus removal data for Protein A and anion exchange chromatography steps from five recombinant protein purification processes to support continued viral clearance during repeated use of chromatography columns.
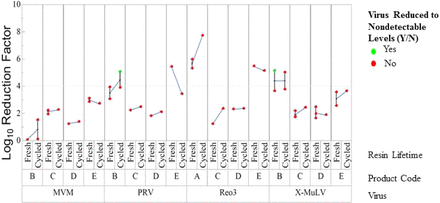
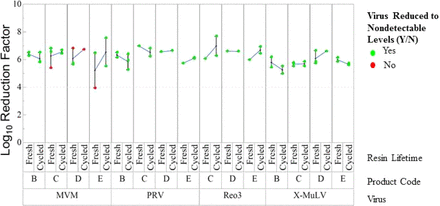
Protein A and anion exchange chromatography steps were studied in six and eight resin lifetime studies, respectively, where columns were cycled at least 100 times at a small scale to simulate resin lifetime. In all studies, columns were tested for removal of up to four model viruses exhibiting diverse physical and chemical properties at the beginning (fresh resin) and at the end of resin lifetime (cycled resin). Selected viruses were xenotropic murine leukemia virus (X-MuLV), minute virus of mice (MVM), reovirus type 3 (Reo3), and pseudorabies virus (PRV). Lifetime studies included traditional Protein A ligand on different base beads and alkali-stable affinity ligand. Figure 1 (Protein A) and Figure 2 (anion exchange) show the average clearance of fresh and cycled resin for each product and virus combination connected by lines. For both resin types, the standard deviation for virus clearance within fresh or cycled resin replicates was 0.6 log10, and the variation was the greatest when replicates were not tested concurrently. This observation highlights the importance of controlling the comparison of fresh and cycled resin to ensure that any change in performance is attributable to resin performance rather than testing artifacts (e.g., virus stock purity, virus titer, virus assay).
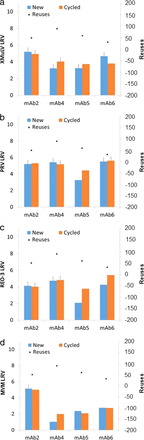
Clearance of four model viruses by fresh and cycled Protein A chromatography resin.
Clearance of four model viruses by fresh and cycled anion exchange chromatography resin.
Virus removal by cycled resin was generally equal to or greater than fresh resin, and a decrease exceeding 1 log10 over resin lifetime was observed for only one virus–monoclonal antibody (mAb)–column combination. Maintained viral clearance over resin lifetime was predicted by stable trends for relevant process performance attributes, such as yield and Chinese hamster ovary (CHO) host cell protein (HCP) for Protein A and CHO DNA and CHO HCP for anion exchange. These data support that end of resin life testing for virus clearance could be eliminated for resins where relevant process performance attributes are maintained throughout the intended number of cycles.
Review of Viral Clearance Capacity of Used Chromatography Resins In Biologics Purification Processes
Glen Bolton, Amgen
Amgen historical viral clearance data for cycled chromatography resins was summarized, and the analysis was similar to analyses that have been performed by other companies previously (2). The data included 60 comparisons of new versus cycled chromatography resins, with results from eight different recombinant monoclonal antibodies. The log reduction value (LRV) was measured using the following four viruses: MVM, X-MuLV, PRV, and Reo3. Chromatography resins included Protein A, cation exchange (CEX) chromatography, flow-through mixed-mode anion exchange chromatography (MM-AEX), and bind–elute hydrophobic interaction chromatography (HIC). The data show that column cycling does not negatively affect the viral clearance capability of the resins.
Overall, 56 of 60 comparisons of LRV values between new and used resins were within 1 log. Of the four with >1 log difference, none had a difference of 2 logs or more, three showed higher LRV values for cycled resin, and one showed higher LRV value (1.2 logs higher) for new resin. The single case where the LRV was higher for new resin was not indicative of a general trend and was likely an outlier caused by the variability of the viral assay.
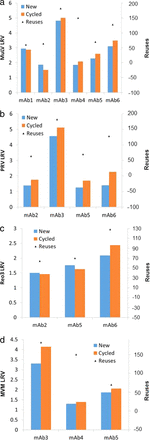
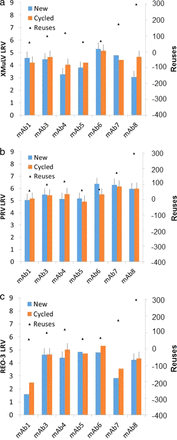
The Protein A chromatography data showed that all 16 antibody comparisons were within 1 log (Figure 3). Previously, a measurement of MVM clearance using Protein A with a protein fused to the constant domain (Fc) of an antibody showed 1.2 logs lower clearance for used resin than for new resin. Again, this decline was likely an outlier caused by the variability of the viral assay. All measurements showed detectable virus.
Comparison of new versus cycled Protein A chromatography: (a) xenotropic murine leukemia virus; (b) pseudorabies virus; (c) reovirus 3; and (d) mouse minute virus.
The CEX bind–elute chromatography data showed that all 22 comparisons were within 1 log. Thirty-one pool measurements showed no detectable virus (Figure 4).
Comparison of new versus cycled CEX chromatography. “|” indicates no detectable virus. (a) Xenotropic murine leukemia virus; (b) pseudorabies virus; and (c) reovirus 3.
The MM-AEX flow-through chromatography data showed that 13 of 16 antibody comparisons were within 1 log. Three measurements showed between 1 and 2 logs of higher clearance of used resin. Sixteen pool measurements showed no detectable virus. The data are presented in Figure 5.
Comparison of new versus cycled MM-chromatography. “|” indicates no detectable virus. (a) Xenotropic murine leukemia virus; (b) pseudorabies virus; (c) reovirus 3; and (d) mouse minute virus.
The HIC bind–elute chromatography data showed that all four antibody comparisons were within 1 log (Figure 6). Seven pool measurements showed no detectable virus.
Comparison of new versus cycled HIC chromatography LRV values for X-MuLV and PRV for two antibodies. “|” indicates no detectable virus.
In summary, viral clearance data from new and cycled chromatography resins at Amgen show that cycling does not negatively affect the viral clearance capability of chromatography steps. These data are consistent with previously published studies (3⇓⇓–6) and show limited value for end of resin lifetime viral clearance studies.
Evaluation of Virus Carryover and Lifetime In Non-platform Process
Shohei Kobayashi, Chugai Pharmaceutical Co., LTD
The virus carryover and virus partitioning in virus-spiking study by using AEX, CEX, and hydrophobic interaction (HI) and multi-mode (MM) chromatography resins, used for non-platform antibody processes, are summarized in Table I.
Summary of Viral Clearance and Virus Carryover in Different Chromatography Modes
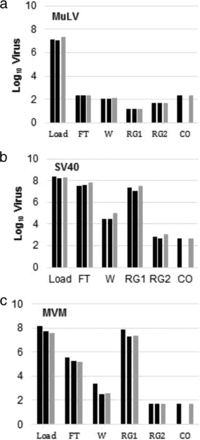
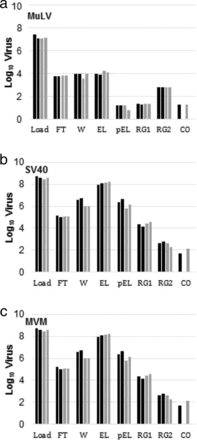
In general, it is well known that AEX can be an effective viral clearance step under the flow-through mode. Although AEX is used as bind and elute for our pipeline with a certain amount of salt in the elution buffer, the effective viral clearance was still confirmed for three different model viruses (MuLV, Simain Virus 40 (SV40), and MVM), as shown in Table I. In addition, column aging does not affect viral clearance or product quality, as shown by the data published by other companies.
For the case of HI and MM using Capto Adhere chromatography resin, effective or moderate MuLV removal was observed. The data of virus partitioning profile show that the retention of MuLV is stronger than that of antibody in the column. However, MVM and SV40 reductions are less or are not observed, and the elution behavior is similar to that of antibody under these chromatography modes (Figures 7 and 8). It was indicated that the hydrophobicity on the surface of MuLV, enveloped virus, makes the removal effective in HI and MM. Like AEX and CEX, the column aging more than 100 cycles does not affect viral clearance. Furthermore, 1 M of NaOH as a regeneration solution works well for virus removal and inactivation, and no virus carryover is observed in all tested conditions.
Log10 viral loads from virus partitioning in multi-mode flowthrough chromatography. (a) MuLV; (b) SV40; and (c) MVM. *Below detection limit. Black: naïve resin; gray: aged resin; FT: flow-through; W: wash; RG1: regeneration 1; RG2: regeneration 2; CO: carryover.
Log10 viral loads from virus partitioning in multi-mode bind–elute chromatography. (a) MuLV; (b) SV40; and (c) MVM. *Below detection limit. FT: flow-through; W: wash; EL: elution; pEL: post elution; RG1: regeneration 1; RG2: regeneration 2; CO: carryover.
Virus Clearance By Chromatograph: Resin Lifetime and Carryover Studies
Dayue Chen, Lilly
While the primary function of chromatography unit operations in downstream purification processes is to remove process- and/or product-related impurities such as DNA, HCP, and product aggregates, these unit operations are often evaluated in viral clearance studies to ensure adequate overall viral clearance capacity of the downstream processes. As a reminder, the ICH Q5A(R1) guideline (1) clearly states that, “Over time and after repeated use, the ability of chromatography columns and other devices used in purification scheme to clear virus may vary. Some estimate of the stability of the viral clearance after several uses may provide support for repeated use of such columns.” Further, the guideline continues, “Assurance should be provided that any virus potentially retained by the production system would be adequately destroyed or removed prior to reuse of the system. For example, such evidence may be provided by demonstrating that the cleaning and regeneration procedures do inactivate or remove virus.”
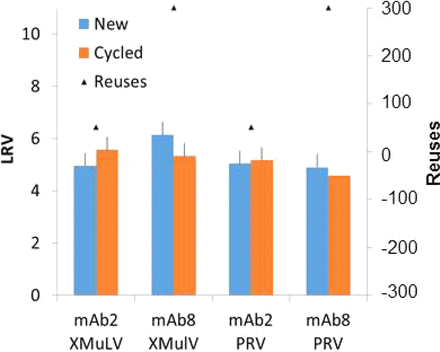
The Protein A chromatography unit is used to provide additional virus removal capacity in the Lilly platform purification process. To meet the regulatory expectations, we have carried out specific studies to assess whether resin subjected to repeated virus removal is affected by repeated cycles (up to hundreds) of Protein A resin cleaning and regeneration throughout the resin lifetime. Specifically, spike and recovery experiments were performed to determine the virus removal capacity by Protein A chromatography column packed with either new resin or lifetime resin. As shown in Table II, lifetime resin consistently provided comparable or even slightly better virus clearance as measured in log10 reduction factors (LRF). In addition, there were no noticeable differences in virus-partitioning profiles between the new and lifetime resins.
Viral Clearance Comparison Between New and Lifetime Protein A Resin
We also assessed the ability of Protein A column regeneration procedure (1% phosphoric acid + 1% acetic acid for 60-min contact time) for destroying or removing viruses. We reasoned that Protein A columns are only repeatedly exposed to known endogenous retrovirus–like particles (RVLP). The probability of processing adventitiously viral-contaminated bioreactor harvests undetected is highly unlikely owing to extensive in-process monitoring and testing in good manufacturing practice (GMP). Therefore, the risk of using Protein A resins that have been exposed to adventitious viruses, such as MVM, is extremely low. As noted in the ICH Q5A(R1) (1): “Cell lines such as CHO, C127, BHK, and murine hybridoma cell lines have frequently been used as substrates for drug production with no reported safety problems related to viral contamination of the products.” Based on the aforementioned rationale, we justified the use of XMuLV as the only model virus to assess whether the regeneration procedure adequately eliminates retroviruses and thus prevents RVLP accumulation and carryover. Specifically, a typical such evaluation involved two back-to-back Protein A chromatography runs. In the first run, XMuLV was spiked into cell-free bioreactor harvest prior to column loading. Upon the completion of the first run, the column was subjected to the regeneration procedure. In the second run, the cell-free bioreactor harvest was directly loaded onto the regenerated column without virus spiking. Samples from all individual fractions of the two runs were analyzed by cell-based infectivity assay. If the regeneration procedure effectively eliminated any potentially retained XMuLV following the completion of the first run, then no XMuLV would be detected in any of the fractions from the second run. As shown in Table III, no XMuLV was detected in any of the fractions from the second run. These results provide adequate assurance that reuse of Protein A column following the standard resin regeneration process poses no risk for retrovirus accumulation and carryover.
Effectiveness of Resin Regeneration Procedure in Inactivating/Removing Retroviruses
In summary, our results from multiple molecules show that virus clearance by Protein A is not adversely affected by up to 300 cycles of lifetime reuse. Consistently comparable or better viral clearance has been shown using lifetime resin from multiple molecules. The column regeneration procedure provides consistent and reliable retrovirus inactivation, as no carryover of retrovirus has ever been detected. Our data presented here are consistent with those reported by others in the past (4, 7, 8).
Our data indicate that it is entirely viable to use in-house data and prior experience/knowledge to support future regulatory filings without carrying out molecule-specific studies with regard to resin reuse and virus carryover. The overall risk associated with such approach seems negligible.
Retrospective Evaluation of Cycled Resin In Viral Clearance Studies—a Multiple Company Collaboration
John Mattila, Regeneron Pharmaceuticals Inc.; Mike Clark, AbbVie Inc.; Justin Weaver and Jeremy Pike, Alexion; Bradford Stanley, Biogen; Deqiang Yu, Bristol-Myers Squibb; Eileen Wilson and Olga Galperina, GlaxoSmithKline plc; Xinfang Li, ImmunoGen Inc. (now of MabPlex); David Roush and Scott Tobler, Merck, Sharp and Dohme, Inc.; Sherrie Curtis, Roche; Andreas Flicker and Johanna Kindermann, Shire plc; Norbert Schuelke, Takeda; Richard Whitcombe, UCB
Considerable resources are spent within the biopharmaceutical industry to perform viral clearance studies, with resin cycling and subsequent viral clearance studies being particularly time- and resource-intensive.
The BioPhorum Development Group Viral Clearance Working Team performed a collaborative retrospective analysis to evaluate cycled resin performance for two commonly used types of resin in biopharmaceutical manufacturing: Protein A chromatography and AEX chromatography. The analysis of virus clearance for a cycled resin included seven parameters for Protein A chromatography and eight parameters for AEX chromatography (bind/elute and flow-through operations). Key variables evaluated in the assessment included virus type, product load amount, resin reuse, and virus loading amount.
The extensive data set presented provides a reference point for understanding the impact of resin cycling on virus clearance capability and would have been difficult to generate for any individual company. The data are valuable in evaluating the necessity of performing viral clearance studies for cycled Protein A and AEX chromatography resins. The BioPhorum Development Group Viral Clearance Working Team intends to share the complete retrospective analysis in a future publication.
Summary
The experience on stable or slightly increasing virus reduction with reuse of Protein A was extended by four presentations in this session. The BioPhorum Development Group Viral Clearance Working Team presented a compilation of virus reduction data during purification of 19 molecules by Protein A chromatography, and only two exceptions were noted. This is also in line with experience from >50 European marketing authorization procedures. In addition to two exceptions (one with PRV and one with X-MuLV), there was only one molecule where three of four model viruses showed significant (>1 log) decrease of LRV. However, used resin was studied three years later, and in the same study with used resin, LRV was also observed with the other columns, raising questions about the comparability of study conditions. As concluded in the previous viral clearance meetings, it is advisable to compare old and new resin carefully side by side.
As from the previous Viral Clearance Symposium, it was noted that the Center for Drug Evaluation and Research considers virus reduction by Protein A chromatography robust with respect to resin age and has developed internal guides to this effect for reviewers (2). The need to perform virus reduction studies with reused Protein A resin was also discussed during the Joint Biologics Working Party/Quality Working Party Workshop with stakeholders in relation to prior knowledge and its use in regulatory applications at the European Medicines Agency. It was concluded that omission of virus reduction studies with used Protein A could be acceptable if prior knowledge has been published in scientific journals and/or in the viral clearance meeting reports (9).
Data from other chromatographic columns from all of the presenters also showed consistent virus reduction between new resin and cycled resin. Such studies are usually performed for marketing authorization, and the reuse of resin has been qualified by acceptable reduction of impurities (i.e., HCP and DNA) in the context of marketing authorizations. Nevertheless, it would still be desirable to have more experience with impurities as surrogates for virus reduction (i.e., whether breakthrough of these impurities occurs later than breakthrough of various model viruses). These data would be used to justify if the viral clearance study for cycled use is needed.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2018