Abstract
Rapid oxidation of polysorbate 80 in histidine buffer was observed upon brief exposure to stainless steel. Liquid chromatography–mass spectrometry analysis indicates degradation of both polyoxyethylene sorbitan and polyoxyethylene head groups and unsaturated fatty acid chains, with further confirmation by reversed-phase high-performance liquid chromatography data. Both Fe2+ and Fe3+ were shown to induce polysorbate 80 oxidation. The degree of oxidation in polysorbate 20 and polysorbate 80 are comparable for the head groups and saturated fatty acid esters. However, the same phenomenon was not observed with placebo or monoclonal antibody at a threshold protein concentration, formulated in sodium citrate, in combination with histidine and sodium citrate, or with Na2 ethylenediaminetetraacetic acid (EDTA). Further, polysorbate 80 oxidation was not observed with Lilly's antibody containing the active ingredient LY2951742, at or above a threshold concentration. Finally, no major polysorbate 80 degradation was observed in histidine buffer, with or without protein, in containers composed of glass or plastic, or when stainless steel exposure was otherwise completely absent. Finally, the 2-oxo oxidation form of histidine was not observed, but the other oxidation products and modifications of histidine were identified.
LAY ABSTRACT: Rapid oxidation of polysorbate 80 in histidine buffer was observed upon brief exposure to stainless steel. The degree of oxidation in polysorbate 80 and polysorbate 20 were comparable. However, the same phenomenon was not observed with placebo when formulated in sodium citrate, in combination with histidine and sodium citrate, or with Na2 ethylenediaminetetraacetic acid (EDTA). Polysorbate 80 oxidation was not observed with Lilly's antibody containing the active ingredient, LY2951742, at or above a threshold concentration. No major polysorbate 80 degradation in histidine buffer was observed when stainless steel contact was completely absent.
- Polysorbate 80
- Polysorbate 20
- Histidine
- Metal ion
- Stainless steel
- Oleic acid
- Lauric acid
- Citrate
- Oxidation
- Monoclonal antibody
Introduction
The development of monoclonal antibody (mAb) drug formulations for a variety of clinical applications is quite attractive due to their disease-state specificity and potential for a decreased level of side effects relative to synthetically derived therapeutics. Development of commercially viable antibody pharmaceuticals has not always been straightforward. This is due to the variable behavior of antibodies, even though they possess similar structures. In attempting to address some of the challenges encountered in the development of antibody therapeutics, Harris et al. reviewed the commercial-scale formulation and characterization of therapeutic recombinant antibodies (1). In a different review, antibody production and purification were discussed (2). Use of polysorbate 80 or polysorbate 20 in large-molecule parenteral formulations has become a standard approach to stabilize the molecule from stress-induced events, which could lead to aggregation or degradation. In recent times, the use of disposable bag technologies for manufacture of biologics is on the increase, but stainless steel remains the preferred choice for many manufacturing organizations. An area of increasing concern and scientific scrutiny is the potential contamination of drug products by leachables entering the product from a variety of container types during manufacturing and storage (3). The instability of Immunoglobulin G (IgG) mAbs induced by redox-active metal ions and photo-irradiation has been reported in the literature (4). A number of excipients used in the drug product formulation have the potential to undergo process- or storage-related degradation due to events such as contact with surface(s) and/or with mixing elements during agitation or mixing steps of the manufacturing process or during storage in various container types. The end-result of such interaction(s) has the potential to degrade the active ingredient and subsequently affect one or more of the drug product quality attributes, such as purity or potency. Two such excipients, histidine buffer and polysorbates, have the potential to undergo oxidation reactions, and the evaluation of such reactions has been studied extensively in the literature. The oxidation of histidine has been extensively studied and reported in the published literature (5⇓–7). Similarly, the degradation mechanism of polysorbates have also been extensively studied and reported in the published literature (8⇓–10).
Based on internal data and published literature on the degradation mechanism of polysorbate 80, a series of studies were conducted to assess the metal-catalyzed oxidation of polysorbate 80 in histidine buffer by studying the impact of different contact materials, surfactant types, metal chelator/antioxidant, buffer types, and protein concentration. Polysorbate 80 degradation was monitored using reversed-phase high-performance liquid chromatography (RP-HPLC) and liquid chromatography–mass spectrometry (LC/MS) assays, and histidine degradation was monitored using an LC/MS assay.
Materials and Methods
Materials and Equipment
LY2951742 antibody is a product of Eli Lilly and Company. Five milliliter (5 mL) Type I borosilicate glass vials and bromobutyl serum stoppers, glass prefilled syringes, and bromobutyl-coated plungers were obtained from the West Company (Exton, PA). Ferric chloride anhydrous was obtained from Sigma Aldrich (St. Louis, MO), disodium ethylenediaminetetraacetate dihydrate (Na2EDTA) was obtained from J.T. Baker (Paris, KY), ferrous sulfate heptahydrate was obtained from Sigma Aldrich, and L-histidine and L-histidine hydrochloride monohydrate were obtained from Kyowa Hakko (Hofu City, Japan). An Agilent HPLC system was equipped with a UV detector and column heater, HPLC column ACE 5 C18-300, 4.6 × 250 mm, 5 μm, P/N ACE-221-2546, weighing was conducted using a Mettler Toledo analytical balance capable of measuring at least 0.01 g, pH measurements were performed on a Thermo Orion (Boston, MA) pH meter with readout to at least 0.01 pH units, 0.2 μm filters [polyvinylidene difluoride (PVDF) or polyether sulfones (PES)] were obtained from Millipore (Burlington, MA), heating block or oven obtained from Fisher Scientific (Chicago, IL), eppendorf adjustable-volume and positive-displacement pipettes were obtained from Fisher Scientific, and centrifugation was conducted using an eppendorf centrifuge obtained from Fisher Scientific.
Methods
Liquid Chromatography to Detect Polysorbate 80 Degradation
A stability-indicating reversed-phase HPLC method, using a gradient with UV absorbance detection at 195 nm, was used for testing the polysorbate 80 samples. Polysorbate 80 determination involves hydrolysis of the sample using base and heat to liberate oleic acid, followed by quantitation of total oleic acid (TOA) against an internal standard curve. The standard curve is prepared in the same manner from polysorbate 80 to stoichiometrically determine the amount of polysorbate 80 present via the oleic acid marker. This quantified value will also include any free oleic acid (FOA) present in solution prior to the hydrolysis step of the sample preparation. The second analytical property, FOA, is quantified against the same standard curve. The FOA sample is prepared without the use of the hydrolysis step, determining only the total amount of FOA in solution. Polysorbate 80 content is calculated as the difference between the TOA in the hydrolyzed sample and the FOA in the non-hydrolyzed sample.
LC-MS Assay to Detect Polysorbate Degradation
Polysorbate 80 or 20 degradation was analyzed by LC-MS injecting 1 μL of undiluted solution or 5 μL of diluted solution. A Waters (Milford, MA USA) Acquity UPLC coupled to ThermoFisher (Bremen, Germany) Velos Orbitrap Elite mass spectrometer was applied for all stability sample analysis. Separations were performed on a Varian PLRP-S reversed-phase column (1 × 50 mm, 1000Å, 5 μm) at 80 °C using 0.05% trifluoroacetic acid (TFA) in water as mobile phase A and 0.04% TFA in acetonitrile as mobile phase B. The column was equilibrated at 8% mobile phase B for 1 min, linearly increased from 8% to 100% over 14 min, held at 100% for 1.5 min, then re-equilibrated at 8% mobile phase B. Eluting peaks separated at 0.2 mL/min between 2 to 16.5 min for the stability samples without antibody or between 2 to 4.5 min and between 6.2 to 16.5 min for samples with antibody were analyzed using an electrospray ionization (ESI) source operating at positive model, scan range of 160 to 2000 amu, Fourier transform mass spectrometry resolution at 120,000, spray voltage of 4.0 kV, capillary temperature of 275 °C, sheath gas of 40 °C, and source induce collision of 50 V.
Polysorbate 80 or 20 levels were quantitated by LC-MS based on the understanding that dioxalanylium ion intensities are proportional to the amount of intact polysorbate. The peak area of each polyoxyethylene sorbitan fatty acid ester was obtained from the extracted ion chromatogram of its dioxalanylium ion and added to give the peak area of intact polysorbate 80 or 20. The relative percent of intact polysorbate 80 or 20 was calculated by comparing its peak area with that of the sample at time zero (100%) or the control sample (only buffer and polysorbate 80 or 20) at each time point (100%). Relative percent of each individual fatty acid ester or different-order ester, such as mono-ester or higher-order ester (di- or more ester), could be calculated similarly.
LC-MS Assay to Detect Histidine Degradation
Samples of polysorbate 80 or 20 in histidine solutions were analyzed by LC-MS, injecting 1–2 μL of undiluted solution. A Waters Acquity UPLC (H class) coupled to Waters Xevo G2-XS mass spectrometer was applied for all sample analysis. Separations were performed on a ThermoFisher Hypercarb™ Porous Graphitic Carbon LC Column (2.1 × 150 mm, 3 μm particle size) at 30 °C using 0.05% TFA in water as mobile phase A and 0.04% TFA in acetonitrile as mobile phase B. The column was equilibrated at 0% mobile phase B for 2 min, linearly increased from 0 to 20% over 12 min, from 20 to 100% over 5 min, held at 100% for 2 min, then re-equilibrated at 0% mobile phase B after a zig-zag wash step. Eluting peaks, separated at 0.2 mL/min between 2 to 20 min, were analyzed using an ESI source operating at positive model, scan range of 50 to 2000 amu, spray voltage of 3.2 kV, sampling cone of 100.0, cone voltage of 30.0 V, source temperature of 150 °C, desolvation temperature of 300 °C, and desolvation gas flow (L/h) of 900.0.
Experimental: Sample Preparation for Various Studies
The samples were prepared using MilliQ water; filled into glass vials, passivated/cleaned 316 L stainless steel containers, or disposable bags; and stored at an accelerated temperature of 25 °C. The solutions were maintained between pH 5.5 to 6.0, with a majority of the samples also containing sodium chloride as a tonicity agent to simulate the drug product matrix.
A. Effect of Contact Material on Polysorbate 80/Polysorbate 20 Degradation
For the initial study involving various contact surfaces, 10 mM histidine buffer, 150 mM sodium chloride, and 0.05–0.06% (w/v) polysorbate 80 at a pH range 5.5–6.0 was prepared and exposed for up to 7 days at 25 °C to disposable bag, stainless steel surface, and stainless steel with nitrogen overlay. After 7 days of contact, all samples were subsequently transferred into glass vials and stored for an additional 30 days at 25 °C. Sample aliquots were removed at periodic intervals and tested for polysorbate 80 degradation.
For the study involving contact with a prefilled syringe system containing a staked stainless steel needle, two buffer formulations were prepared: 10 mM histidine buffer, 150 mM sodium chloride, 0.05–0.06% (w/v) polysorbate 80 at pH range 5.5–6.0; and 10 mM histidine buffer, 150 mM sodium chloride, 100 ppm Na2EDTA, and 0.05–0.06% (w/v) polysorbate 80 at pH 6.0. The two buffer formulations were filled into prefilled syringe systems and stored at 25 °C, with periodic samples removed and submitted for polysorbate 80 and histidine assays over a 28 day period.
In order to compare the degradation profiles of polysorbate 20 with polysorbate 80, another study was designed using two identical buffer formulations but with different surfactants: 10 mM histidine buffer, 150 mM sodium chloride, 0.05–0.06% (w/v) polysorbate 80 at a pH range 5.5–6.0; and 10 mM histidine buffer, 150 mM sodium chloride, and 0.05–0.06% (w/v) polysorbate 20 at a pH range 5.5–6.0 were stored in stainless steel containers at 25 °C for a period of 15 days and assayed for surfactant degradation.
An additional study was designed to understand if the buffer and/or polysorbate 80 concentration had an impact on polysorbate 80 degradation. The four formulations listed below were prepared and exposed to stainless steel surfaces for a period of 2 weeks at 25 °C, and samples were removed and submitted for testing.
50 mM histidine, 150 mM sodium chloride, 0.06% (w/v) polysorbate 80, pH range 5.5–6.0.
50 mM histidine, 150 mM sodium chloride, 0.01% (w/v) polysorbate 80, pH range 5.5–6.0.
5 mM histidine, 150 mM sodium chloride, 0.06% (w/v) polysorbate 80, pH range 5.5–6.0.
5 mM histidine, 150 mM sodium chloride, 0.01% (w/v) polysorbate 80, pH range 5.5–6.0.
B. Effect of Metal Ion, Chelator, and Antioxidant on Polysorbate 80 Oxidation
In order to understand the effect of metal ions, chelators, and antioxidants on polysorbate 80 oxidation, a buffer matrix consisting of 10 mM histidine buffer, 150 mM sodium chloride, 0.05–0.06% (w/v) polysorbate 80 at a pH range 5.5–6.0 was used. For the metal ion study, the buffer matrix was spiked with 0.5 ppm of Fe2+ and stored at 25 °C for 4 weeks. Samples were pulled at periodic intervals and submitted for testing. In order to understand the effect of a chelator like Na2EDTA on polysorbate 80 oxidation, the specified buffer matrix was spiked with 5 ppm of Fe2+ and divided into two containers. One container was spiked with Na2EDTA solution, while the other container remained un-spiked. The stability of these solutions was monitored over a 3 month duration at a storage temperature of 25 °C. In order to assess the effectiveness of an antioxidant on preventing polysorbate 80 oxidation, 1 mM methionine was added to the histidine buffer matrix, followed by spiking with 5 ppm of Fe2+. This formulation was stored at 25 °C, and samples were removed for periodic testing over a period of 4 weeks.
C. Effect of Buffer Type on Polysorbate 80 Oxidation
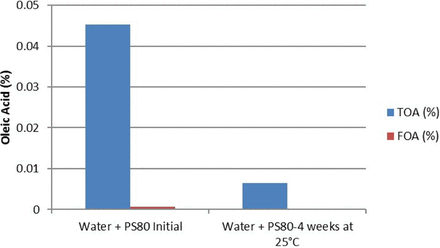
In order to assess if the buffer type played a role in polysorbate 80 oxidation, identical concentrations of polysorbate 80 were prepared in histidine and citrate buffer matrices and exposed to stainless steel. These solutions were subsequently transferred to glass vials and stored over a period of several months at 5 °C and 25 °C and submitted for testing. As a follow-up study, in order to assess if buffer species had a role in polysorbate 80 oxidation, a polysorbate 80 solution was prepared in water and exposed to a stainless steel surface over a 4 week period at a storage temperature of 25 °C and submitted for testing.
D. Effect of LY2951742 Antibody Concentration on Polysorbate 80 Degradation
In order to assess if antibody concentration played a role in polysorbate 80 degradation, LY2951742 antibody was prepared at 5, 40, 70, 100, and 120 mg/mL in 10 mM histidine buffer, 150 mM sodium chloride, at a pH range of 5.5–6.0 with a polysorbate 80 concentration of 0.05–0.06% (w/v). The solutions were filled into glass prefilled syringes with staked stainless steel needles and placed on stability for up to 10 months at the storage temperatures of 5 °C and 25 °C.
Results and Discussion
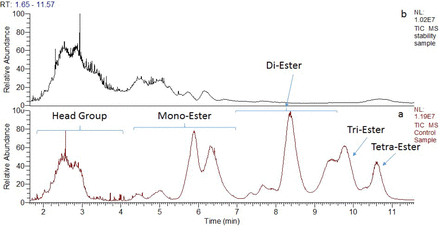
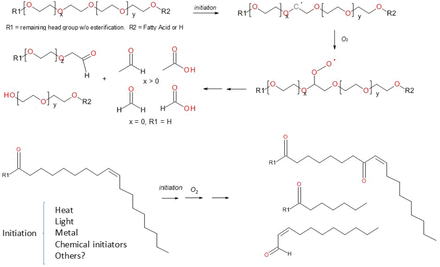
Total ion chromatograms (TICs) of LC/MS analysis for the stressed histidine buffer with polysorbate 80 are presented in Figure 1. After stressing at 25 °C, most of the polysorbate 80 was degraded and shifted to an early elution. According to mass spectrometry characterization and the literature (10, 12⇓⇓–15), the degradation mechanism for polysorbate 80 is well known and is presented in Figure 2. Polysorbate 80 oxidation consists of two oxidation pathways. The head group of the non-ionic surfactant is oxidized by the free radical species, resulting in fragmentation and generation of acetaldehyde, formaldehyde, acetic acid, formic acid, and several fragments of the head group. At the same time, the free radical species oxidizes any unsaturated fatty acids attached to the head group, attacking any sites of unsaturation within the fatty acids and leading to various degradation and fragmentation species.
Total ion chromatograms (TIC) of LC/MS analysis for the histidine buffers with polysorbate 80 stressed at 5 °C (a) and 25 °C (b) for 6 months in glass vials.
Proposed degradation mechanism for polysorbate 80.
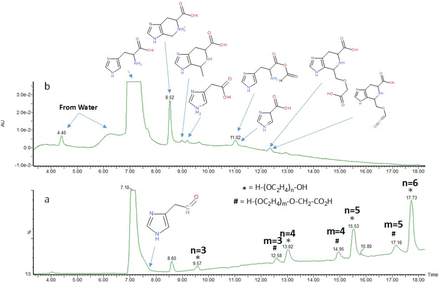
The stressed histidine buffer with polysorbate 80 was subjected to LC/MS analysis, and the LC-UV chromatogram and TIC are shown in Figure 3. Based on accurate mass and tandem spectrometry analysis, more than 20 histidine degradation products were identified; the major ones are presented in the figure. All degradation products were produced from the two types of degradation pathways. The major degradation pathway involves the attack of aldehydes (acetaldehyde, formaldehyde) generated from head group oxidation of polysorbate 80 to generate several histidine modifications. Histidine oxidation is the other degradation pathway, but the 2-oxo-histidine species was not detected.
Total ion chromatogram (TIC) (a) and LC-UV chromatogram (b) of LC/MS analysis for 10 mM histidine buffer with polysorbate 80 stressed at 25 °C for 6 months in glass vials. The major histidine and PEG degradation products identified with accurate masses and tandem mass spectrometry are labeled in the figure.
Effect of Contact Material on Polysorbate 80/Polysorbate 20 Degradation
The contact materials study results are presented in Table I. The effect of stainless steel and nitrogen on stability after storage for 30 days at 25 °C is presented in Table II.
Effect of Contact Material on Polysorbate 80 Degradation, 7 days at 25 °C1
Stability Data in Glass Vials at 25 °C1
As shown in Table I, no major change in the polysorbate 80 content by RP-HPLC was observed in glass control, disposable bag, and stainless steel container with nitrogen overlay for up to 7 days at 25 °C. These samples were clear and colorless upon visual inspection. However, the buffer solution contained within the stainless steel container exhibited a dramatic decrease in the level of polysorbate 80 after 7 days of storage at 25 °C and was visibly pale yellow, suggesting oxidation of polysorbate 80 and/or modification of histidine. Data for histidine controls without polysorbate 80 did not show any color change (data not shown). The dramatic decrease in polysorbate 80 for this sample was confirmed by LC-MS, showing only 1.9% of intact (i.e., un-oxidized) oleic acid, or a nearly complete oxidation of oleic acid after 7 days of incubation with stainless steel at 25 °C compared to a nominal level of 75% of intact oleic acid for freshly prepared solutions. Additionally, the buffer solution in the stainless steel container with nitrogen overlay was clear and colorless upon visual inspection, consistent with the glass control and disposable bag samples. To further study this behavior, the sample in the stainless steel container with nitrogen overlay was transferred into a glass vial, sealed, and stored for an additional 30 days at 25 °C. Analysis of the 30 day sample for polysorbate 80 content and appearance revealed the complete loss of polysorbate 80, and the solution was no longer clear and colorless but had become visibly pale yellow. This data suggests that nitrogen overlay briefly delayed the oxidation of polysorbate 80 and/or modification of histidine while retaining solution clarity. However, oxidation of polysorbate 80 continued to occur on stability.
Assessment of solutions prepared with degraded polysorbate 80 showed lower surface tension than solutions prepared with fresh polysorbate 80, but the overall critical micelle concentration (CMC) of polysorbate 80 did not change as a result of polysorbate 80 degradation (data not shown). These data suggest that degraded polysorbate 80 may still possess surfactant properties.
The results from the prefilled syringe needle soaking study are presented in Figures 4 and 5. The polysorbate 80 oxidation was monitored by using HPLC to measure TOA and FOA, and histidine degradation was monitored by measuring the intact histidine in the exposed samples.
Effect of prefilled syringe needle contact on polysorbate 80 oxidation.
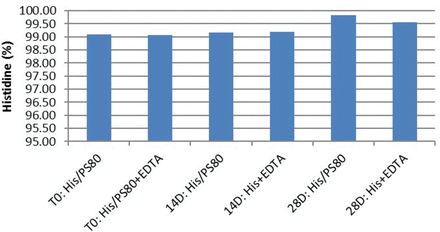
Effect of prefilled syringe needle contact on histidine degradation.
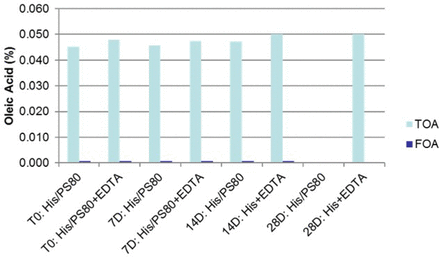
As expected, no total oleic acid (intact) was measured on day 28, suggesting oxidation of polysorbate 80. However, the TOA content remained intact for the Na2EDTA-containing samples, indicating that an iron chelator, such as Na2EDTA, functions as an antioxidant by scavenging reactive oxygen species (ROS), which are potentially responsible for the formation of free radicals (11). This data was further confirmed by the low-to-no detected level of FOA. Further, analysis of the samples for histidine degradation indicated no significant degradation of the histidine species.
Based on the observed degradation of polysorbate 80 in histidine buffer upon brief exposure to stainless steel, an evaluation of surfactant type was conducted. As both polysorbate 80 and polysorbate 20 have the same sorbitan head group but differ only in the fatty acid chain compositions, the impact of stainless steel contact on polysorbate 20/histidine combination was assessed with polysorbate 80/histidine as a positive control. The results of the study are presented in Table III.
Effect of Stainless Steel on Polysorbate 80/20 Oxidation, 15 Days at 25 °C1
Consistent with the previous data, the polysorbate 80 content was reduced to 0.005% from the initial content of 0.06% over the 15 day time period at 25 °C, indicating significant polysorbate 80 oxidation. This data was further confirmed by the visual appearance of the solution, turning from clear and colorless to a pale yellow solution. Although no change in visual appearance was seen with the polysorbate 20/histidine buffer solution over the 15 day study duration, the percent oxidation of the saturated fatty acid content was comparable to that of the polysorbate 80/histidine buffer solution. In addition, the observed amount of histidine modification was comparable between the two surfactants. Overall, these data suggest similar trends of degradation between polysorbate 80 and polysorbate 20 for the head groups and saturated fatty acid esters in histidine buffer upon exposure to stainless steel, supporting the impact of the formation of free radicals. However, the major component of polysorbate 80 is oleic acid. Oxidation of oleic acid was substantial during the exposure studies, most likely resulting in a pale yellow color.
The study results for the evaluation of different histidine and polysorbate 80 ratios on polysorbate 80 oxidation upon exposure to stainless steel are presented in Table IV.
Effect of Stainless Steel on Histidine and Polysorbate 80 Ratio on Polysorbate 80 Oxidation, at 25 °C1
As shown, no major trend with respect to polysorbate 80 oxidation was seen with any of the conditions. By the end of the study duration, 2 weeks at 25 °C, a significant loss in polysorbate 80 was observed with all the ratios evaluated compared to initial ratios, suggesting that the oxidation of polysorbate 80 was independent of the histidine/polysorbate 80 ratio. No changes in solution color or clarity were observed throughout the study duration. Iron levels (Fe2+ and Fe3+) measured by Integrated Coupling Plasma (ICP) were at or below the limit of detection, 50 ppb.
A. Effect of Metal Ion, Chelator, and Antioxidant on Polysorbate 80 Oxidation
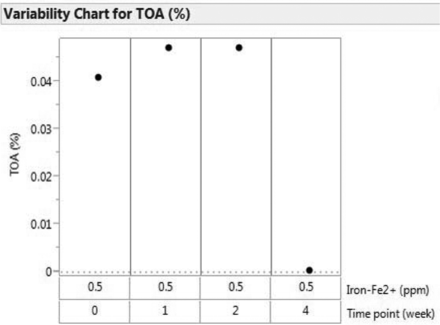
The results from Fe2+ spiking, with and without Na2EDTA and methionine, are presented in Figure 6 and in Tables V, VI, and VII.
Effect of metal ion on polysorbate 80 oxidation in glass vials.
Effect of Na2EDTA on Polysorbate 80 Oxidation, 3 Days at 25 °C1
Effect of Na2EDTA on Polysorbate 80 Oxidation, 3 Months at 25 °C1
Polysorbate 80 Oxidation in Histidine Buffer in the Presence of Spiked Iron and Methionine1
The data as presented in Figure 6 and Tables V, VI, and VII indicate no significant polysorbate 80 oxidation in 5 ppm Fe2+-spiked samples when Na2EDTA was present. However, there appeared to be no beneficial effect of methionine in slowing down polysorbate 80 oxidation. In addition, samples containing Na2EDTA remained stable for up to 3 months at 25 °C, whereas samples that did not contain Na2EDTA were stable only for 3 weeks, beyond which noticeable oxidation of polysorbate 80 started to occur. The LC/MS data in Table VII demonstrates that methionine was not effective in preventing polysorbate 80 oxidation because the amount of oxidation and the oxidized forms of the unsaturated fatty acid, oleic acid, were comparable with and without methionine in the presence of spiked Fe2+. This data supports the proposed degradation mechanism involving the formation of free radicals and does not support an adverse impact of headspace oxygen on stability. Other possible explanations could be that an insufficient concentration of methionine was in the solution to bind the level of free radicals present or that methionine has no impact on the type of free radicals (i.e., hydroxyl, alkyl, sulfate, etc.) formed during stainless steel exposure.
B. Effect of Buffer Type on Polysorbate 80 Oxidation
The results from a head-to-head study comparing the oxidation of polysorbate 80 in histidine and sodium citrate buffer solutions during exposure to stainless steel are presented in Figures 7 and 8. The polysorbate 80 oxidation was monitored by measuring the total oleic acid content on stability using the HPLC assay.
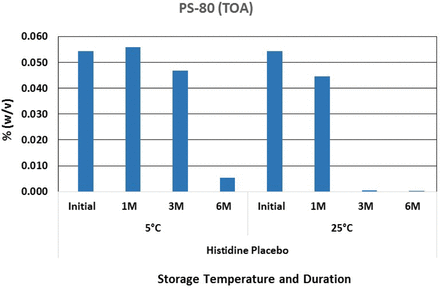
Polysorbate 80 oxidation in histidine buffer in glass vials.
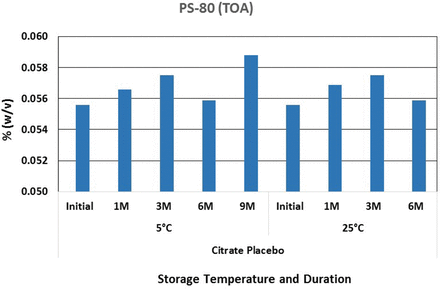
Polysorbate 80 oxidation in sodium citrate buffer in glass vials.
Consistent with the Na2EDTA data, no significant oxidation of polysorbate 80 was observed in sodium citrate buffer. This could result from sodium citrate acting as both a weak chelator and a scavenger of the ROS that are responsible for the formation of free radicals. However, oxidation of polysorbate 80 in histidine buffer started to occur after storage for as little as 1 month at 25 °C and 3 to 6 months at 5 °C. A follow-up study was conducted by preparing polysorbate 80 solutions in water with the solution exposed to stainless steel. The results are presented in Figure 9.
Polysorbate 80 oxidation in water in glass vials.
The results presented in Figure 9 show that polysorbate 80 undergoes oxidation, even when present in water, after exposure to stainless steel. The data demonstrate that free radicals play a significant role in polysorbate 80 oxidation in histidine buffer or water (absence of buffer), irrespective of the concentration of the buffer and/or polysorbate 80. Further, the data indicate that once the free radicals are formed, metal ions such as Fe2+ can act as an initiator to further accelerate the reaction. The free radical formation can be prevented by the addition of a weak chelator such as sodium citrate or a strong chelator such as Na2EDTA. Although it is difficult to prevent the histidine + polysorbate 80 buffer solution from coming in contact with stainless steel or other metals during the manufacturing process, moving to a completely stainless steel–free system such as glass or disposable bag technology may be an option to minimize stainless steel contact. Alternatively, the addition of a chelating agent can be considered as an option to minimize or prevent polysorbate 80 oxidation.
C. Effect of LY2951742 Antibody Concentration on Polysorbate 80 Degradation
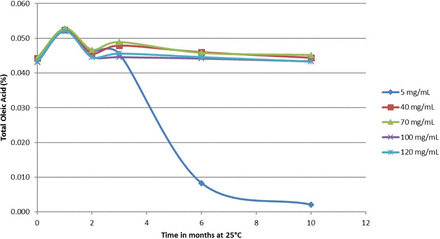
The results of LY2951742 antibody concentrations in histidine buffer in prefilled syringes are presented in Figure 10.
Polysorbate 80 oxidation in LY2951742 monoclonal antibody as a function of concentration in prefilled syringes at 25 °C.
As shown in Figure 10, data for the 5 mg/mL LY2951742 antibody formulation in histidine buffer in contact with prefilled syringes with a stainless steel staked needle showed a loss of approximately 75% of TOA after 6 months of storage at 25 °C and nearly complete loss of TOA after 10 months of storage at 25 °C. Identical formulations of LY2951742 antibody at concentrations higher than 40 mg/mL in the same histidine buffer matrix did not show any loss of TOA over the same time period, supporting the observation that mAb concentration plays a significant role in minimizing and/or preventing the loss of TOA upon exposure to stainless steel at the accelerated temperature, 25 °C. These results demonstrate the protective effect of proteins, with concentration playing an even more critical role in preventing polysorbate 80 oxidation in the LY2951742 formulations. We hypothesize that the protective effect of mAbs may result from stabilization or quenching of free radicals, thus preventing or minimizing polysorbate oxidation and/or histidine degradation. The threshold for protein concentration for minimizing polysorbate 80 oxidation at 5 °C was generally lower compared to accelerated temperature (data not shown). These results demonstrate the role of protein concentration on polysorbate 80 or polysorbate 20 oxidation and the importance of understanding the relationship between protein type/concentration, stainless steel exposure, and polysorbate 80 oxidation.
For the LY2951742 antibody formulation, sodium citrate buffer was not considered a viable option due to suboptimal stability performance (data not shown). Additionally, the use of Na2EDTA to prevent polysorbate 80 oxidation at LY2951742 concentrations below the threshold level was not considered a viable option due to the significant chelating potential of Na2EDTA for Si (syringe lubricant), which could affect the functionality of the prefilled syringe system upon long-term storage.
Conclusions
The root cause of polysorbate 80 degradation observed in histidine buffer was determined to be metal-catalyzed oxidation. Both Fe2+ and Fe3+ were capable of inducing polysorbate 80 oxidation. The LC/MS analysis confirms oxidation of the sorbitan and fatty acid portions of polysorbate 80 in the histidine-containing buffer solution, with confirmation from HPLC data. The same behavior was not observed with buffer solutions containing sodium citrate alone or histidine buffer containing Na2EDTA or sodium citrate. Elevated levels of Fe2+ and/or Fe3+ and accelerated temperature appear to accelerate the process of polysorbate 80 degradation. In addition, improperly prepared stainless steel surfaces, excessive surface area to volume ratio, and excessive light exposure may also contribute to polysorbate 80 oxidation. The degradation mechanism appears to be independent of the ratio between histidine and polysorbate 80, solution pH, or buffer concentration. Although surface tension of solutions containing degraded polysorbate 80 were lower than solutions containing freshly prepared polysorbate 80, the overall CMC of polysorbate 80 does not change as a result of polysorbate 80 degradation based on limited data (data not shown). The degree of oxidation in polysorbate 20 and polysorbate 80 is comparable for the head groups and saturated fatty acid esters. Substantial oxidation of oleic acid in polysorbate 80 may be the contributing factor for the pale yellow color upon exposure to stainless steel. The polysorbate 80 oxidation occurred rapidly in buffer (placebo) and slower in LY2951742 antibody drug product, suggesting the protective effects of protein. The data supports the hypothesis of a threshold level for LY2951742 for prevention of polysorbate 80 oxidation. If a low protein concentration formulation is desired in histidine buffer, then use of histidine buffer containing Na2EDTA may be explored as a possible solution to the issue. Alternatively, switching to citrate buffer may also be a viable option. However, before implementing any of these options, the stability of the formulation should be evaluated. No major polysorbate 80 degradation was observed in histidine buffer (with or without protein) in glass containers or in plastic bags where the contact of stainless steel was completed eliminated. Finally, the 2-oxo oxidation form of histidine was not observed, but the other oxidation products and modifications of histidine were identified.
Conflict of Interest Declaration
The authors declare that they have no competing interests related to this article.
Acknowledgments
The authors would like to thank Dr. Natarajan Rajagopalan for providing technical guidance and thorough review of the manuscript.
- © PDA, Inc. 2018