Abstract
In the vapor-phase hydrogen peroxide (VPHP)-sanitized environment, VPHP uptake by product-contacting components could eventually lead to undesired oxidation of biological drug products. Silicone tubing and primary packaging materials are prominent examples of such product-contacting surfaces that are typically processed/sterilized prior to use. This study investigated the VPHP-uptake tendency of these components and how their respective processing/sterilization methods affect the uptake behaviors. Silicone tubing that was sterilized via autoclave or gamma irradiation exhibited different VPHP uptake patterns—decreased uptake rates post autoclaving vs. increased uptake rates post gamma irradiation. The reduced uptake tendency of autoclaved tubing is maintained for 14 days after sterilization, whereas the uptake tendency of irradiated tubing was mostly reversed to normal levels 1 month after irradiation. Empty glass vials adsorbed hydrogen peroxide via the diffusion of VPHP into the vial with high vial-to-vial variability. Vial pretreatment (i.e., depyrogenation) and surface hydrophilicity/hydrophobicity impacted the uptake tendency. Stoppers and empty syringes also adsorbed hydrogen peroxide but at a relatively low level. The uptake behavior of these components appeared to correlate with water levels at the surface (i.e., hydrophilicity). This study provides process development scientists and engineers an in-depth understanding of the VPHP uptake by critical product-contacting surfaces so that they can mitigate the impact on drug product quality.
LAY ABSTRACT: This study investigated vapor-phase hydrogen peroxide (VPHP) absorption by biopharmaceutical drug products via VPHP uptake by critical product-contacting components during the aseptic manufacturing process with a focus on various pretreatments and processing of these components. Sterilization of silicone tubing by gamma irradiation or autoclaving resulted in different VPHP uptake profiles with different effect durations. Primary packaging components, such as vials, syringes, and stoppers, also showed different levels of VPHP uptake with surface hydrophilicity/hydrophobicity playing a critical role. These outcomes suggested that VPHP uptake is a complex phenomenon and should be carefully considered to minimize its impact on product quality. The approach and outcome of this study can benefit scientists and engineers who develop biological product manufacturing processes by providing an in-depth understanding of drug product process risks.
- Vapor-phase hydrogen peroxide
- Monoclonal antibody
- VPHP uptake
- Primary packaging components
- Silicone tubing
- Sterilization
- Gamma irradiation
- Autoclave
- VHP
- Vaporized hydrogen peroxide
1. Introduction
Processing and packaging facilities providing aseptic filling of biopharmaceutical drug product (DP) into sterilized containers require consistent methods of cleaning and sanitization to ensure the removal of microorganisms. Vapor-phase hydrogen peroxide (VPHP) is a common and highly effective sanitizing agent against spores, bacteria, and viruses (1⇓⇓–4). A general concern for manufacturing biopharmaceutical products in a VPHP-sanitized environment is the migration of residual VPHP into the DP solution in the form of hydrogen peroxide (H2O2), because H2O2 is an effective oxidizing agent that is known to oxidize proteins (5⇓⇓⇓–9).
The mechanism in which residual VPHP enters the DP solution during production is critical technical knowledge that can guide proper manufacturing process controls to ensure that VPHP uptake levels in final DP containers (for example, vials, prefilled syringes, and so forth) are within acceptable limits. Hubbard and Eppler (10) provided an overview of potential H2O2 uptake sources in the isolator and the restricted access barrier system (RABS) filling lines. They identified four likely sources that may eventually lead to H2O2 absorption by the DP solution: empty glass surfaces (vials or syringes); the exposed product at the tip of the filling needle; open (unstoppered), filled vials/syringes; and the tubing of the filling line (10).
When considering two of the identified sources, that is, filled vials/syringes and the exposed product at the tip of the filling needle, it is well-known that H2O2 is absorbed by the DP solution directly from the atmosphere. However, the mechanisms driving the transfer of H2O2 from the product-contacting components (i.e., empty vials, syringes, and tubing) to the DP solution are not yet understood. The product-contacting tubing flow path is considered the most prominent source owing to the high surface area-to-volume ratio. The primary packaging components (i.e., vials, syringes, and stoppers) are the final contact surfaces with the DP solution, and these components may have a more direct impact on product quality.
Most DP filling lines use platinum-cured silicone tubing in the product flow path, especially in peristaltic filling systems, because of its advantageous mechanical properties (11–12). Silicone tubing (polydimethylsiloxane) is highly permeable to low-molecular-weight species and nonpolar substances (12, 13). VPHP is a low-molecular-weight compound and can easily permeate the tubing wall into the DP solution via diffusion and partitioning. A recent study by Hubbard and coworkers (14) demonstrated that VPHP uptake by the silicone tubing in a peristaltic filling line inside a VPHP-decontaminated isolator plays a critical role in H2O2 migration into the DP. However, there are various aseptic operations in which flow path components are sterilized by various methods and assembled in the filling barrier systems before or after H2O2 sanitization.
Pharmaceutical tubing is typically sterilized by autoclaving (steam sterilization), gamma or electron-beam (e-beam) ionizing irradiation, or ethylene oxide (EtO) gas treatment (15, 16). The choice of sterilization method should consider factors beyond the sterilization efficacy, including the impact on tubing properties, the effect on product quality, and the operation logistics (17⇓⇓⇓–21). If the flow path (tubing) assembly is set up before production, the tubing and associated parts are typically sterilized by autoclaving or steam sterilization at the manufacturing site. For preassembled tubing systems, the assembly is typically presterilized by irradiation (usually gamma irradiation) at a contract manufacturing location and subsequently shipped to the DP manufacturing site. Because autoclaving and gamma irradiation are the most commonly used tubing sterilization methods, we investigated how these two methods would affect the tubing’s VPHP uptake tendency.
It is less well-known whether primary packaging components adsorb H2O2 from VPHP as they are made of various materials—typically glass for vials and syringes and rubber for stoppers—and their surfaces may be coated to minimize the interactions with the DP (22⇓⇓–25). Like the tubing, these primary packaging components are processed (i.e., washed and sterilized) before being filled with the DP solution. In this study, we evaluated the H2O2 uptake tendency of these components and determined their potential impact on the DP quality.
2. Materials and Methods
The components used in this study are summarized in Table I. They include tubing, glass vials, syringes, and stoppers that were either pretreated (or sterilized) in-house or at a contract laboratory before the VPHP uptake experiments.
Components used in VPHP uptake Experiments
2.1 Components Pretreatment or Sterilization
Tubing was sterilized by either autoclaving or gamma irradiation as listed in Table I and as described in Section 2.2.1. Glass vials were pretreated by washing and depyrogenating or conditioning in a humidity chamber. Stoppers were pretreated by autoclaving.
2.2 VPHP Uptake Experiments
A Pharmaceutical Safety Isolator (PSI-M, Skan AG) was used to test the filling components in a controlled VPHP environment. In addition to normal isolator functionality, this isolator is able to maintain a constant H2O2 concentration in the atmosphere by continuously vaporizing H2O2 while all other functions of the isolator are in normal production mode. By changing the dosing rate and the concentration of the H2O2 stock bottle, any VPHP level can be targeted and maintained. This level was monitored by a Picarro H2O2 sensor (Model G1114 or G2114).
All uptake experiments were performed inside the isolator. The concentration of absorbed H2O2 was determined using a qualified Amplex UltraRed Hydrogen Peroxide assay.
2.2.1 VPHP Uptake by Silicone Tubing:
To test H2O2 absorption by silicone tubing, a PD12I pump head and an MC12 pump controller (Flexicon Corp.) were placed outside the isolator. Sample tubing of a predetermined length was assembled into the pump head and run into the isolator through triclamp fitting ports. The two ends of the sample tubing ran outside the isolator through separate fitting ports; one end was connected to a water bottle (upstream) and the other to a filling needle (downstream). The section of sample tubing inside the isolator could be exposed to various concentrations of VPHP. The sample tubing was sterilized either by autoclaving or by exposure to gamma irradiation. Control tubing, which was not sterilized by either method, was processed similarly to, and tested side by side with, its respective sterilized counterpart.
For sterilization by autoclave, the silicone tubing (1.6 mm internal diameter × 1.6 mm wall thickness) was autoclaved at 121°C for 60 min following a predefined program ending with a drying step. The autoclaved tubing was then cut to a predetermined length and exposed to 100 ppb VPHP in the isolator. VPHP exposure was performed immediately or days (e.g., 2 and 14 days) after autoclaving. Each piece of tubing was then primed with ultrapure water and incubated in the VPHP environment for up to 60 min before a water sample was taken for Amplex testing.
For sterilization by gamma irradiation, the silicone tubing (3.2 mm and 1.6 mm internal diameter × 1.6 mm wall thickness) was sterilized with 40 kGy of gamma irradiation in the original sealed plastic bag. Samples of the 3.2 mm irradiated tubing were exposed to 100 ppb VPHP in the isolator 3 and 8 days after irradiation. After 29 days, the bag of 1.6 mm irradiated tubing was opened and exposed to 100 ppb VPHP in the isolator. During each VPHP exposure, the samples of tubing were primed with ultrapure water and incubated in the isolator for various durations up to 90 min before a water sample was taken for Amplex testing.
2.2.2 VPHP Uptake by Glass Vials:
To test H2O2 adsorption by glass vials, open vials were introduced into the isolator via the rapid transfer port (RTP) after the VPHP in the isolator reached a constant level of 500 ppb. The vials were positioned upright on the floor in the center of the isolator. After VPHP exposure for various time intervals, the vials were removed from the isolator via the RTP. Each VPHP-exposed vial was immediately pipetted with ultrapure water (or water hereafter) and stoppered with a clean stopper that had not been exposed to VPHP. The amount of water added was vial size-dependent: 0.5 mL into 2 cc vials, 1.0 mL into 6 cc vials, 1.5 mL into 15 cc vials, 2.0 mL into 20 cc vials, and 3.0 mL into 50 cc vials. Each vial was rolled on its side and turned upside down so that water would contact all interior surfaces for complete H2O2 absorption in the water. All vials were left upside down in a dark environment for 1 h before being tested using the Amplex UltraRed assay.
2.2.3 VPHP Uptake by Glass Syringes:
To test H2O2 adsorption by glass syringes, ready-to-use syringes with a staked in needle and a rigid needle shield were used. These open syringes were introduced into the isolator via the RTP after VPHP in the isolator reached a constant level of 500 ppb. Syringes stood with the tip down in a stand on the floor in the center of the isolator. After VPHP exposure for various time intervals, the syringes were removed from the isolator via the same RTP. Each syringe was immediately pipetted with 0.35 mL of ultrapure water and stoppered using a hand stoppering tool with a clean plunger stopper that had not been exposed to VPHP. Each syringe was turned end-to-end for a few rotations and shaken vigorously to ensure all interior surfaces had been in contact with the water. Syringes were left on their side in a dark environment for 1 h before testing. The contents of the syringe were expelled into a 2 cc vial and analyzed using the Amplex UltraRed assay.
2.2.4 VPHP Uptake by Butyl Rubber Stoppers:
To test H2O2 adsorption (or absorption) by butyl rubber stoppers, the stoppers were introduced into the isolator via the RTP after VPHP in the isolator reached a constant level of 500 ppb. All stoppers were placed on the floor of the isolator with the product-contacting side facing upward. After VPHP exposure for various time intervals, the stoppers were removed from the isolator via the RTP. Each VPHP-exposed stopper was immediately seated onto a clean, unexposed 2 cc or 6 cc vial filled with 0.5 mL or 1.0 mL of ultrapure water, respectively. Each vial was then turned upside down to allow the water to be in full contact with the stopper. Stopper-containing vials were placed upside down in a dark environment for 1 h before the water sample was tested for H2O2 concentration using the Amplex UltraRed assay.
2.3 Determination of H2O2 Concentration in Water
H2O2 concentrations in water were measured using the fluorometric Amplex UltraRed assay. This method has been previously described (26).
3. Results and Discussion
3.1 VPHP Uptake by Silicone Tubing: Effect of Sterilization on Uptake Behaviors
We previously reported a VPHP uptake case study involving isolator operation (14). To minimize human manipulation, the entire filling flow path, including the silicone tubing, was preassembled in the isolator before VPHP sanitization. The flow path was sterilized via clean-in-place (CIP) and steam-in-place (SIP) processes during isolator aeration after the decontamination phase. In this case, the tubing assembly absorbed a substantial amount of VPHP during the decontamination phase; however, the absorbed H2O2 was carried away by water for injection and steam and was depleted after CIP/SIP. The VPHP uptake behavior of the tubing assembly was eventually dictated by the residual VPHP concentration in the isolator atmosphere during the filling operation.
In another barrier filling option, featuring a RABS, the liquid flow path components would be sterilized in advance and then assembled on the filler (post VPHP decontamination and aeration) immediately before the start of the filling operation. In this case, the tubing components would be autoclaved (or steam-sterilized) at the filling site shortly (within a few days) before filling. If a preassembled single-use filling line is used, the tubing assembly is typically gamma irradiated by the vendor and shipped to the manufacturing site. Thus, gamma-irradiated tubing may be used for manufacturing weeks (or months) after sterilization.
3.1.1 Tubing Sterilized by Autoclaving:
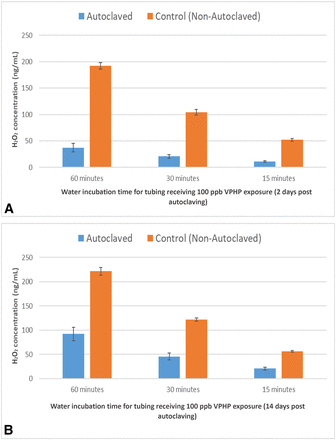
The autoclaved tubing absorbed substantially less H2O2 than the control (∼20% of the control) when the VPHP uptake experiment was performed 2 days after autoclaving (Figure 1A. When the VPHP uptake experiment was performed 14 days after autoclaving, the uptake tendency of the autoclaved tubing recovered somewhat (to 39%; Figure 1B) relative to the control. Since autoclaved tubing is typically used within a few days after sterilization, it is expected that the autoclaving process would present a favorable condition with less H2O2 absorption.
(A) VPHP uptake into silicone tubing (1.6 mm ID) 2 days after autoclaving. Tubing was primed with water and exposed to 100 ppb VPHP in the isolator for 30, 60, and 90 min before sampling and Amplex testing; (B) VPHP uptake into silicone tubing (1.6 mm ID) 14 days after autoclaving. Tubing was primed with water and exposed to 100 ppb VPHP in the isolator for 30, 60, and 90 min before sampling and Amplex testing.
This decreased VPHP uptake tendency of the autoclaved tubing might be because of the reduced water content in the tubing as a result of the drying process at the end of the autoclaving cycle. Silicone tubing is permeable to gas molecules owing to free volume or “openings” created in association with the high flexibility (movement) of the silicone–oxygen chain in silicone (27). Free volume permits gas diffusion. Because silicone is hydrophobic, tubing permeability can be decreased if water is removed from the free volume of the tubing material.
Additional studies were performed to validate this hypothesis. In the first experiment, the autoclaved tubing was filled with water overnight to allow for water reabsorption before the 100 ppb VPHP uptake experiment. The autoclaved tubing showed an increase in VPHP uptake but it was not fully recovered (∼42% compared to the control; Table II).
Impact of hydration and dehydration on VPHP uptake of autoclaved and nonautoclaved Tubing
Two studies were performed to assess the impact of dehydration of nonautoclaved tubing on VPHP uptake behaviors. Tubing dehydration took place by either incubation at 130°C for 12 h or by vacuum drying. The dehydrated tubing showed reduced VPHP uptake tendency, at 50%–60% of the control, but not to the level of the autoclaved tubing (Table II). This suggested that the water content in the tubing does impact VPHP uptake behaviors, but that other unknown factors associated with autoclaving also play a role in the reduced tendency of VPHP uptake.
3.1.2 Tubing Sterilized by Gamma Irradiation:
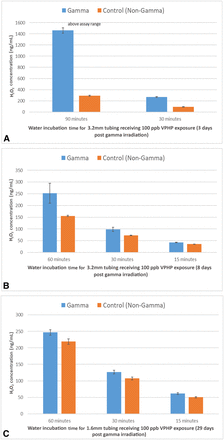
Three days after irradiation, the 3.2 mm tubing was tested and it absorbed substantially greater amounts of H2O2 than the control (Figure 2A). The H2O2 level for the irradiated tubing was three times higher than that of the control after a 30 min incubation. The difference after the 90 min incubation was even more significant, but the amount of H2O2 absorbed in the irradiated tubing was unknown as the detected concentration of H2O2 exceeded the qualified range of the assay.
(A) VPHP uptake into silicone tubing (3.2 mm ID) 3 days after gamma irradiation. Tubing was primed with water and exposed to 100 ppb VPHP for 30 and 90 min before sampling and Amplex testing; (B) VPHP uptake into silicone tubing (3.2 mm ID) 8 days after gamma irradiation. Tubing was primed with water and exposed to 100 ppb VPHP for 15, 30, and 60 min before sampling and Amplex testing; (C) VPHP uptake into silicone tubing (1.6 mm ID x 1.6 mm wall) 29 days after gamma irradiation. Tubing was primed with water and exposed to 100 ppb VPHP for 15, 30, and 60 min before sampling and Amplex testing.
In a separate experiment performed 8 days after irradiation, another sample of the irradiated tubing was removed and evaluated for VPHP uptake in the same manner except for having three incubation groups at 15, 30, and 60 min. Interestingly, the irradiated tubing still absorbed more H2O2 than its nonirradiated counterpart but to a lesser extent when compared with the tubing tested 3 days after irradiation (Figure 2B), indicating that the impact of irradiation on the VPHP uptake rate is reversible. This observation was confirmed in a third experiment with smaller tubing (1.6 mm internal diameter × 1.6 mm wall thickness), which was left in the original sealed bag until being tested for VPHP uptake 29 days after irradiation. In this final experiment, the amount of H2O2 absorbed by the irradiated and the nonirradiated tubings were almost identical for each incubation time (Figure 2C).
Overall, these data suggest that gamma irradiation alters the H2O2 absorption behaviors of silicone tubing; however, the effects are reversible approximately one month after irradiation. The DP manufacturer may not be aware of this phenomenon; from the perspective of manufacturing scheduling and shipping, it is unlikely that the vendor-irradiated tubing will be used for manufacturing within 30 days of irradiation.
Changes in the mechanical properties of irradiated silicone tubing have been reported. Gamma irradiation is known to induce changes in the molecular architecture of silicone rubber by increasing its molecular weight (cross-linking) and decreasing its elasticity (16). Higher doses of gamma irradiation and longer treatment cycles have been shown to cause higher cross-link densities (28). However, the relationship between the enhanced permeability and the increased cross-link structure of gamma-irradiated silicone tubing is difficult to comprehend, in particular, because the increased permeability is reversible, whereas the cross-linked structure is permanent. Mazor and Zilberman (29) reported a decrease in the water vapor transmission rate with an increased irradiation dose for a wound dressing consisting of a barrier layer made of poly(DL-lactic-co-glycolic acid). Although in that case the gamma irradiation still increased the polymer cross-linking, they attributed the increased water vapor transmission rate to the formation of microfractures in the polymer matrix (i.e., creation of pores allowing for better water vapor permeation). If this phenomenon is applicable to irradiated silicone, the same hypothesis can explain the increased H2O2 permeability but not the reversibility behavior.
3.2 VPHP Uptake by Empty Glass Vials
3.2.1 Adsorption of H2O2 on Glass Surfaces:
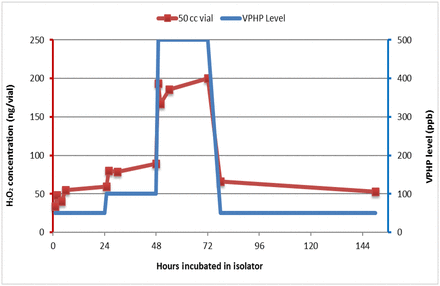
During filling operations, empty vials can sit for hours on the line (while exposed to a VPHP atmosphere) because of process interruptions before being filled. Can an inert surface like glass adsorb H2O2 from the atmospheric VPHP during the filling operation, and how fast and how much can it adsorb? To address these questions, a set of 50 cc vials were placed in the isolator and exposed to three levels of VPHP concentration in the following sequence: 50, 100, 500, and back down to 50 ppb (the blue profile in Figure 3). Three vials were removed at multiple time points within each level of VPHP exposure for incubation with ultrapure water and Amplex testing. The result (red line in Figure 3) showed that glass vials could indeed adsorb H2O2 in a reversible fashion: ∼50 ng/vial while exposed to 50 ppb VPHP, ∼80 ng/vial while exposed to 100 ppb, ∼200 ng/vial while exposed to 500 ppb, and back to ∼50 ng/vial while exposed to 50 ppb. The level of adsorption and desorption closely followed the VPHP concentration (shown as the red profile in Figure 3) and reflected the possible correlation of the surface adsorption by the glass and the VPHP level in the isolator atmosphere.
VPHP uptake by 50 cc glass vials (red) in the isolator in response to three levels of VPHP concentration in the sequence of (1) 50 ppb, (2) 100 ppb, (3) 500 ppb, and back to (4) 50 ppb (blue). Three vials were analyzed by Amplex testing at multiple time points within each level of VPHP exposure.
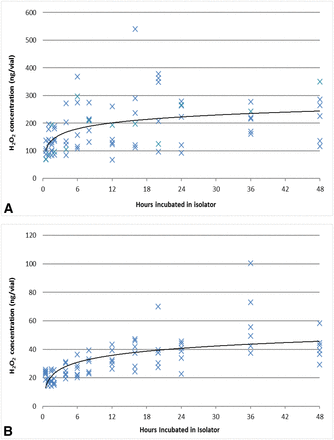
A follow-up study was performed. Empty vials (20 cc and 50 cc) were placed in the isolator and exposed to a VPHP concentration of 500 ppb for up to 48 h. Six vials were removed at each time point for Amplex testing. The correlation of the H2O2 adsorbed to the glass surface and the VPHP exposure time is summarized in Figures 4A (50 cc vials) and 4B (20 cc vials), which display a slight upward trend with a steep initial uptake. After 24 h exposure, the amount of H2O2 adsorbed was in the approximate range of 225 ng per 50 cc vial and 40 ng per 20 cc vial. For oxidation-sensitive proteins with a low fill volume in a large vial, this level of H2O2 concentration is likely to impact the DP quality. However, the overall data were difficult to interpret because of significant vial-to-vial variability. For example, when reviewing the 24 h time point for the 50 cc vial, the average uptake of the six 50 cc vials was 207 ng/vial with the minimum uptake, the maximum uptake, and relative standard deviation (RSD) at 93 ng, 279 ng, and 39%, respectively. This level of variability was also observed in other vial sizes (2, 6, and 15 cc). Excluding the variability of the Amplex assay (∼10% RSD), the data suggested other factors may contribute to this high level of variability.
VPHP uptake by (A) empty 50 cc glass vials and (B) 20 cc glass vials in the isolator after exposure to 500 ppb VPHP for different durations (up to 48 h). Six vials were analyzed at each time point by Amplex testing.
3.2.2 Root Cause of Variability in H2O2 Adsorption by Vials:
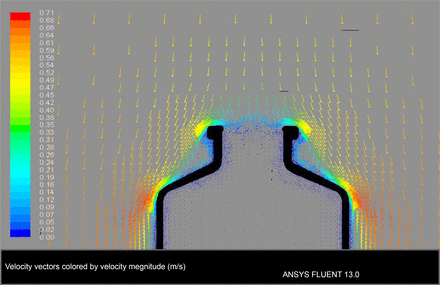
The VPHP uptake variability was initially considered to be related to the airflow pattern in the isolator. Ansys Fluent computer modeling software was used to simulate the airflow inside and around the vial. It assumed downward airflow in the isolator at a velocity of 0.7 m/s (Figure 5). Based on the model, air velocity inside the vial opening was minimal, suggesting that air barely flowed into the vial, and that VPHP would enter the vial mostly through diffusion. Thus, variable H2O2 adsorption on the glass surface should not be attributed to isolator airflow.
Computer modeling simulating isolator airflow inside and around the vial with unidirectional air blowing down toward the vial opening at a velocity of 0.7 m/s.
To minimize procedure-related variabilities, the study (50 cc vials exposed to 500 ppb VPHP) was repeated for a single time point using improved procedures—consistent timing for vial placement in the isolator, vial removal from the isolator, vial incubation, and analytical testing. All vials in the isolator were placed in a predefined zone away from the walls, where airflow might be uneven. There was still a high level of variability, ∼40% RSD (for six vials), in the amount of H2O2 adsorbed by the vials even after the elimination of potential procedural variability. The overall analysis suggested that VPHP uptake variability may be because of heterogeneity of the glass surface for H2O2 adsorption.
3.2.3 Nonuniform Adsorption of VPHP by the Vial’s Glass Surface:
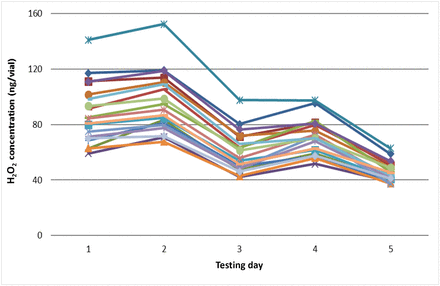
An experiment was performed that subjected 19 vials (50 cc) to repeated VPHP exposure (five times). Before each VPHP exposure in the isolator (500 ppb for 4 h), all 19 vials were preconditioned using the same depyrogenation conditions. All procedures for vial placement, removal, incubation, and analytical testing were executed consistently to avoid procedural variations (see Section 3.2.2). The results demonstrated that each vial showed consistent day-to-day variations; the vial ranking for the amount of adsorbed H2O2 being very similar on each of the 5 days of testing (Figure 6). The same trend was observed for all other vial sizes (data not shown). This shows that VPHP uptake variability is owing to vial variability.
Day-to-day variability of VPHP uptake by 19 empty 50 cc glass vials (each line representing one vial). All vials were preconditioned using the same depyrogenation conditions and the same procedures for vial placement, removal, incubation, and analytical testing to avoid procedural variations.
A hypothesis to explain this vial-to-vial variability may be that the glass surface consists of patches of hydrophilic and hydrophobic zones because of a nonhomogeneous chemical composition. Hydrophilic zones bind water, which increases the vial surface water content and facilitates H2O2 adsorption. Hydrophobic zones discourage water binding, so H2O2 adsorption tendency in these zones is reduced. In Figure 6, the amount of adsorbed H2O2 trends downward with the number of VPHP exposures (and conditioning/depyrogenation cycles) for all 19 vials. The trend is likely because of the high-temperature of depyrogenation during conditioning, which decreased the glass surface’s water content.
To test the hypothesis that vial surface water content dictates the amount of H2O2 adsorbed, five sets of 50 cc vials were placed in the isolator (500 ppb VPHP) for 4 h. The five vial sets are listed below, the control or standard processed vial and then in order of decreasing surface water content:
Group 1. Standard glass vials were washed and depyrogenated (350°C for 3 h). This is standard vial processing prior to filling.
Group 2. Standard glass vials were washed and placed in a humidity chamber (75% relative humidity for 80 h). High-humidity exposure is expected to increase the total surface water content of the vial.
Group 3. Standard glass vials were washed and overdepyrogenated (350°C for 80 h). Extended depyrogenation is expected to decrease the total surface water content of the vial.
Group 4. Vials with hydrophobic coating (SCHOTT TopLyo) were used without processing. The coating increases the hydrophobic zones on the surface and decreases the total surface water content of the vial.
Group 5. Vials with hydrophobic coating (SCHOTT TopLyo) were washed and depyrogenated (350°C for 3 h). Depyrogenation of the coated vial may further decrease the total surface water content of the vial.
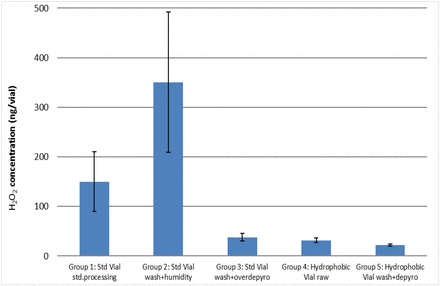
The VPHP uptake results are summarized in Figure 7. Vials in Group 1 served as the control with standard wash and depyrogenation conditioning. As shown in Figure 7, vials with higher surface water content (Groups 1 and 2) adsorbed much higher amounts of H2O2 than the dried (Group 3) and the more hydrophobic vials (Groups 4 and 5). Compared with the control group (∼150 ng/vial), incubating the standard vials in a high-humidity environment (Group 2) facilitated VPHP uptake to ∼350 ng/vial with a high variability (40% RSD), whereas all dried and hydrophobic vials discouraged H2O2 adsorption to below 50 ng/vial and showed low vial-to-vial variability. Drying or depyrogenating vials at a high temperature for a long duration (i.e., extended depyrogenation for Group 3) could severely dehydrate glass surfaces. Applying a hydrophobic coating to vials (Groups 4 and 5) increases hydrophobic zones on the glass surface preventing water from binding to the glass surfaces. All of the data from this experiment support the hypothesis that an increase in glass surface moisture promotes H2O2 adsorption, which exhibits high levels of vial-to-vial variability.
VPHP uptake tendency of 50 cc vials after 4 h exposure to 500 ppb VPHP in the isolator. The five vial groups are listed as the control or standard processed vial, and then in order of decreasing glass surface water content. Each vial group was an average of 10 vials.
3.2.4 Contribution of the Empty Vial to the Overall H2O2 Absorption of the Filled Vial:
A paper calculation based on existing experimental uptake results was performed to evaluate the contribution of uptake in the empty vial to the total H2O2 uptake in a filled vial. The scenario included exposure of empty and filled vials to 100 ppb VPHP isolator air. Under this condition, empty 50 cc vials would adsorb approximately 80 ng per vial (Figure 3). Considering a worst-case low fill volume at 5 mL (10% of the vial size) of a monoclonal antibody formulation, which has a H2O2 limit of 100 ng/mL, the liquid formulation would need to absorb 500 ng of H2O2 before unacceptable H2O2-induced oxidation occurred. In this worst case, H2O2 from empty vials would contribute 16% of the maximum allowable H2O2 absorption. As 50 cc vials are typically filled with >10 mL, the H2O2 contribution from empty vials becomes relatively insignificant.
3.3 VPHP Uptake by Glass Syringes
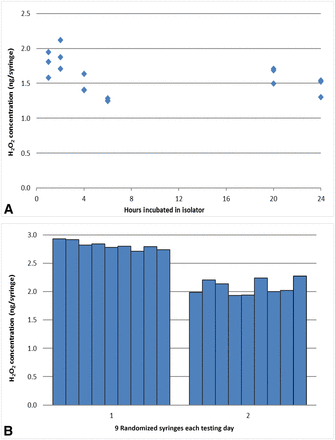
Empty syringes of two sizes, 1 mL and 2.25 mL, were placed in the isolator and exposed to 500 ppb VPHP. The 1 mL long syringes were exposed for up to 24 h with three syringes sampled at each time point for Amplex testing. The results showed very low levels of VPHP uptake for all syringes, in the range of 1–2 ng H2O2 per syringe (Figure 8A), which is below the assay precision. There was no increasing uptake trend with exposure time, and the syringe-to-syringe variability was very low (<10% RSD which is within the range of the Amplex assay). Two sets of 2.25 mL syringes were tested separately on different days (nine syringes on each day) for VPHP exposure for 4 h in the isolator. Consistent results were observed: low uptake (2–3 ng H2O2 per vial) and low syringe-to-syringe variability (Figure 8B).
(A) VPHP uptake tendency by empty 1 mL glass syringes after exposure to 500 ppb VPHP concentration in the isolator for different durations (up to 24 h) with three samples taken at each time point for Amplex testing; (B) VPHP uptake by empty 2.25 mL glass syringes tested separately on two different days (9 syringes on each day) for VPHP exposure of 4 h in the isolator.
The VPHP uptake behaviors of the glass syringes are consistent with those of the hydrophobic vials (Section 3.2.3). The inner surfaces of these glass syringes are indeed hydrophobic as they have been coated with a thin-layer of silicone oil for the purpose of reducing injection force. Given this assessment, the contribution of the empty glass syringe to the overall H2O2 absorption by the DP solution is considered negligible. Syringes requiring no silicone oil coating, such as Crystal Zenith syringes made of cyclic olefin polymer, should be assessed separately for H2O2 absorption/adsorption, which is outside the scope of this study.
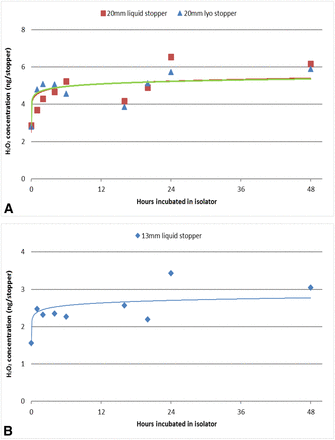
3.4 VPHP Uptake by Butyl Rubber Stoppers
Three types of vial stoppers (20 mm liquid and lyophilization stoppers and 13 mm liquid stoppers) were placed in the isolator and exposed to 500 ppb VPHP for up to 48 h. All stoppers had low levels of uptake, <7 ng H2O2 per 20 mm stopper (liquid and lyophilization) and <4 ng per 13 mm stopper with low stopper-to-stopper variability (<10% RSD) (Figure 9A and B). Again, the amount of H2O2 detected was below the assay precision. All of these stoppers were coated with hydrophobic Flurotec coating, so they showed consistent uptake similar to the hydrophobic glass vials and syringes. The quantity of H2O2 that each stopper absorbed was negligible. Although many rubber stoppers for pharmaceutical applications are coated to minimize stopper–drug interactions, uncoated stoppers should be evaluated separately to understand their H2O2 absorption/adsorption behaviors, which is beyond the scope of this study. In addition, syringe stoppers were not tested in this study, but the same Flurotec coating is expected to yield similar results with negligible VPHP uptake.
VPHP uptake by three types of vial stoppers: (A) 20 mm liquid and lyophilization stoppers and (B) 13 mm liquid stoppers after exposure to 500 ppb VPHP concentration in the isolator for different durations (up to 48 h).
4. Conclusions
Among the product-contacting components investigated in this study, the primary packaging components adsorb H2O2 regardless of their material of construction. Although the primary packaging components may not be considered major contributors to the overall VPHP uptake, their H2O2 adsorption may still present product quality risks and should be assessed based on product-specific configurations. Silicone tubing plays a more critical role and can contribute significantly to overall H2O2 absorption by the DP solution. Silicone tubing displayed unique H2O2 absorption patterns in response to sterilization methods and demonstrated the complicated nature of VPHP uptake behaviors. A common phenomenon shared by all components is that any measure capable of lowering the component water content or making the component surfaces more hydrophobic would reduce their VPHP uptake tendency and minimize adsorption variability. The knowledge gained from this study could help development scientists and engineers prioritize their study strategy and better select product-contacting materials for protein products that are sensitive to H2O2-induced oxidation.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank Dr. Philippe Lam for his work on airflow computer modeling.
- © PDA, Inc. 2019