Abstract
Sterilization is a critical process in the preparation of many drug products. Its execution and validation are addressed in numerous regulatory, pharmacopeial, and industry documents. EMA Annex 1: Manufacture of Sterile Medicinal Products stands alone in giving clear preference to physical measurements over those obtained from biological indicators. This paper reviews principles behind sterilization processes outlining the differences between physical and biological measurements as well as their relationship to each other. The assumptions associated with the use of physical measurement are explored and their derivation from microbiological results is traced with the intent of reaffirming the primacy of biological evidence. The arguments and objections to the use of biological indicators in sterilization are reviewed and deconstructed.
LAY ABSTRACT: Sterilization validation is required by regulatory agencies around the globe. The accepted principles are derived from those originally established in the United States and the United Kingdom during the 1970s. Unfortunately, these have evolved into conflicting expectations. The U.K. placed greater emphasis on physical measurements initially in HTM-10, and this is reflected in the European Medicines Agency's Annex 1 statements for their preeminence over biological data. Practices, primarily in the U.S., give preference to microbiological challenges as confirmation of lethality. This paper reviews sterilization fundamentals and describes the relationship between physical and biological data. It critiques the various arguments for the superiority of physical measurements and supports why microbiological evidence should take precedence.
- Sterilization
- Terminal sterilization
- Biological indicator
- Bioburden
- Probability of a non-sterile unit (PNSU)
- Regulation
- Sterility assurance
- Moist heat
The Belief
The December 2017 draft revision to the European Medicines Agency (EMA) Annex 1: Manufacture of Sterile Medicinal Products reasserts a long-standing position on moist heat sterilization processes that is diametrically opposed to that held by many of the world's sterilization scientists (1): 8.51—Chemical or biological indicators may also be used, but should not take the place of physical measurements.
Identical wording can be found in earlier regulations from the United Kingdom's Medicines Control Agency (now called the Medicines and Healthcare Products Regulatory Agency or MHRA) (2). That the biological indicator is considered a nonessential aspect of the steam sterilization cycle effectiveness contradicts numerous regulatory expectations outside the European Union (3, 4). This publication will review sterilization science to establish whether the EMA stated regulatory expectation is founded in science or is merely opinion-based.
The Premise
Inherent in the EMA's statement is the belief that demonstration of certain physical attributes during a moist heat sterilization process is sufficient evidence that the materials in question have been sterilized. The unstated but implied condition is demonstration of 121°C for 15 min throughout the load as provided in the European Pharmacopoeia (Ph. Eur.) (5). The belief is that demonstration of this physical condition is sufficient to assure effective sterilization.
The Error
The fundamental problem with the preference for physical measurements is the misdirection that it causes. As sterilization processes are intended for the destruction of microorganisms, placing full reliance on physical values implies that they are somehow direct evidence of microbial destruction. That defies reason: the physical measurements must have some original basis in microbial death. The Ph. Eur. stated conditions are likely founded upon some long-lost connection between the stated condition and the reliable destruction of all microorganisms present. Similar statements regarding the secondary role of biological indicators and the reliance on identical sterilizing conditions can be found in the 1983 version of the MHRA Orange Guide (6). The origin of these is equally obscure. The original rationale being lost, it is nevertheless useful to consider the possible evolution of the expected physical conditions.
The Science
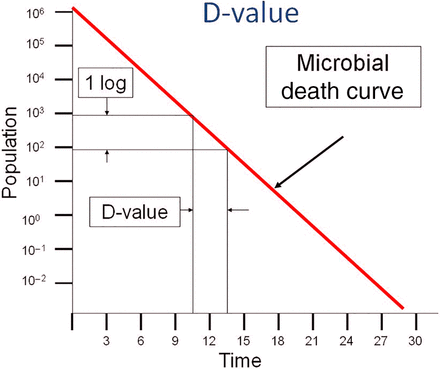
Sterilization processes began to evolve in the late 1700s and the first steam sterilizer was built by Charles Chamberland in 1880 (7). Harriette Chick in the early 1900s was the first to describe the logarithmic order of death for microorganisms, which provides a foundation for modern sterilization science (8). The rate of death in moist heat sterilization is linked to the temperature, with kill occurring more rapidly as the temperature increases. The inverse slope of the logarithmic death curve is the D-value, which is dependent upon a number of factors (e.g., microorganism, substrate, and medium). D-values are commonly determined by fraction-negative or survivor curve studies in which the experimental data allows for calculation of the D-value. The D-value provides a means for estimation of safety levels associated with sterilization processes by determination of the probability of a non-sterile unit (PNSU) (see eq 1).1
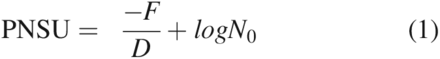
Where PNSU is the logarithmic probability of a non-sterile unit (sterility assurance level or SAL), F is the delivered lethality (equivalent time at reference temperature), D is the D-value of the microorganism, and N0 is the initial population of the microorganism.
A minimum PNSU value is used to provide an added measure of safety to the calculation and additional safety factors can include assuming a high population and extreme resistance of the bioburden present. The F value determined corresponds to the assumed physical conditions of the desired sterilization process. The number of estimations, approximations, and assumptions in the arithmetic determination of the F value for the process are substantial (9) as follows:
Microbial populations are never precisely known;
Microbial resistance is not constant;
Growth media varies in its ability to support growth;
Microbial death curves aren't always straight lines; and
Estimation of the death curve slope is non-exact.
One indication of the inherent variability is the commonly expected tolerance surrounding D value determination of ±20% in the same laboratory (10). Real-world variations in resistance to sterilization are understood to be even greater when different laboratories, different microorganisms, varying substrates, and media variation are all present.
The Ph. Eur. stated norm of 121°C for 15 min throughout the load is equivalent to delivering an F0 of 15 min at all locations. This F value can be inserted into eq 1; however, the other variables (bioburden population and moist heat resistance) necessary for computation of a PNSU are not provided in the Ph. Eur. content, so the expected level of sterility assurance is indeterminate! Although we have a defined physical expectation, there is no means to assess its appropriateness for the destruction of a specific microorganism. In order to determine the minimum PNSU, assumptions regarding microbial population and resistance have to be made. Thus, attaining a standard physical condition establishes that and almost nothing else about the robustness of the actual sterilization process. Confidence in the robustness of a sterilization process meeting only the Ph. Eur. standard cycle expectation is thus inappropriate.2 To determine the PNSU for a moist heat sterilization process, the microbiological population and resistance of the target microorganisms must be known.
Sterilization Process Realities
The individual microorganisms present in a system will die only when they are directly exposed to lethal conditions; however, what those exact conditions are at the point of kill is unknown because physical measurements only describe a portion of what is necessary to establish sterilization efficacy. Microbial death in a moist heat system requires that the microorganism be simultaneously directly exposed to heat and moisture. One could argue that because physical measurements are inherently conservative (especially when safety factors are incorporated into their selection) that validation efforts need only confirm them and that no microbiological evidence is necessary. If only sterilization were that simple. Many factors relating to microbial destruction preclude placing sole reliance on physical measurements for sterilization process validation.
Differences in Measurement Location
The measurement of temperature requires a measuring instrument positioned within the load. Because of size and physical constraints this is not always possible in locations where microbial destruction must be confirmed. Needle lumens, container-closure interfaces, sealed ports, filter cartridges, and other similar configurations force the practitioner to assume that external or adjacent measurements can confirm the presence of sterilizing conditions nearby. Because steam sterilization requires the removal of air and condensate and the introduction of steam at the point of kill, the physical measurements cannot confirm sterilizing conditions where the object's geometry restricts air/condensate egress, steam ingress, and thermal probe placement in these locations. Biological indicators are the only practical and valid approach to study the moist heat sterilization process efficacy in locations that are not suitable for placement of penetration probes.
Presence of Air
One of the acknowledged difficulties with steam sterilization is the potential retention of air within the load items. This received an extraordinary amount of attention from the MHRA and subsequently the EMA, forcing increased attention industry-wide on steam quality and air removal (11, 12). Air is a poor conductor of heat, and its retention in sterilization loads is certainly undesirable. The Bowie–Dick test and its successors endeavor to confirm the sterilizer's performance on a daily basis. These tests, although rigorous, are presumed to emulate air removal and steam penetration in items of very different configuration. Given the diversity of load items, item positioning, and wrapping materials, it is presumptuous to believe that the test results are definitive for all instances. When use of these tests was first pressed by European regulators, one of the principle objections raised by practitioners was the available physical and microbiological evidence of sterilization process efficacy. These were dismissed as inappropriate proof of air removal. Insistence on the standard conditions as definitive evidence of lethality dismissed direct evidence of lethality in favor of circumstantial proof. It should be evident that temperature measurements cannot detect the presence of air retained within the load that would adversely impact sterilization process efficacy, as only microbiological evidence can definitively confirm lethality.
Presence of Superheat
Reliance on physical measurements as confirmation of lethality assumes the presence of saturated steam (13). Superheated steam (steam heated above saturation) can suggest that sterilizing conditions of heat and moisture are present when in fact they are not. Superheated steam is no more effective than dry heat that at temperatures <130°C provides for extremely slow kill (14). As temperature measurements cannot confirm the presence of saturation (the presence of water in the steam), they are inadequate to support sterilization cycle efficacy where superheat might be present.
Changes in Microbial Resistance Due to Substrate Effects
Microbial destruction is understood to be altered by the surface being sterilized. This can be a function of chemistry, permeability, surface roughness, hydrophobicity, and other factors, alone or in combination (9). Measurements made near the surface (as all physical measurements are) cannot fully evaluate the influence these factors will have on microbial destruction. This is particularly true for permeable materials such as filters, fabrics, and elastomeric materials in stoppers, tubing, and gaskets (15, 16).
Measurement Error
Temperature measurement can require penetration of the wrapping materials to position the probes within the load items. Unless managed correctly, the means for probe access can compromise the integrity of the wrapping materials and substantially alter the physical conditions observed. Compromised wrapping when measuring temperatures precludes meaningful results. Similar effects can occur when introducing temperature probes into filled product containers. In these instances, physical measurements in improperly probed load items can differ markedly from those in load units where package/container integrity is maintained. The introduction of temperature probes into prefilled syringes, ampoules, and small vials can alter the container such that the suitability of the results must be questioned (see Figure 1).
Small vial with thermocouple.
As biological challenges can be inserted and recovered without altering the integrity of the monitored item, the measurement errors inherent in temperature monitoring are avoided.
Mistaken Belief in Mathematical Precision
Although not stated, the Ph. Eur. standard cycle conditions are predicated on historical evidence of microbial destruction at similar conditions. This is derived from the work of Chick described previously, which must be understood as a mathematical approximation of the microbial results. The expected cycle incorporates unstated simplifying assumptions about the sterilization process and the undefined microbiological target. Real-world experience with microbiological challenges in sterilization demonstrates that the precepts of a constant rate of kill (D-value), temperature dependence of the kill rate (z-value), and microbiological population (N0) vary substantially. This is certain for biological indicators where the established variation in resistance (which requires knowledge of the parameters mentioned above) is approximately ±20% in the same laboratory (10). Variations with wild-type microorganisms should be understood as even greater. Nevertheless, direct evidence from microbiological results is always preferable to the indirect thermal data.
Nonlinearity of Microbial Death Curves:
As previously stated, the use of physical measurements for proof of sterilization is linked to assumptions regarding the microbial resistance as established through D- and z-value determination. This is often represented by a linear death curve as depicted in Figure 2.
Typical microbial death curve.
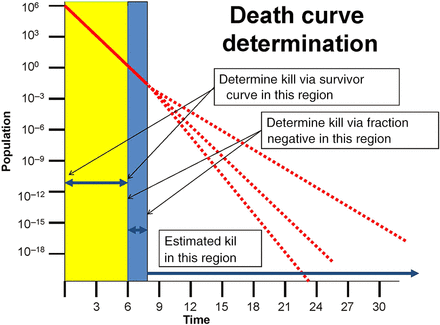
Representing the death curve as a perfectly straight line, although prevalent in most publications, is a simplification of microbiological realities. There is documented evidence of death curves that are decidedly nonlinear including such variants as concave up, concave down, and so forth (9). The existence of these anomalous death curves weakens the argument for physical data correlation with microbiological evidence. The outcome of a microbiologically challenged validation study where the death curve is nonlinear would follow the actual death curve and not the mathematical prediction (see Figure 3).3 Unfortunately, none of this can be confirmed experimentally as the slope of the death curve is unknown beyond the point where viable microorganisms can no longer be recovered. The mathematical estimation of outcome is less reliable than real-world microbiological evidence under those circumstances.
Extrapolated death curves.
There are other objections regarding the use of biological indicators unrelated to the uncertainties of physical measurements. The more commonly indicated concerns are detailed in the following sections.
Contamination Risk from Biological Indicators
One of the objections to the use of biological indicators has been concern regarding potential contamination introduction should the sterilization process be inadequate or a biological indicator lost during the study. In most instances, validation studies entail the placement of biological indicators and thermocouples into the sterilizer where sterilizer loads are prepared, and after completion of the test cycle the load is brought back into that same environment. Accounting for all the biological indicators is readily accomplished. Additionally, nearly all biological indicators used in steam sterilization are wrapped or self-contained, facilitating their location and recovery. The Bacillus and Geobacillus spores commonly used as biological indicators are nonpathogenic and pose no personnel risk. The risks of environmental or personnel contamination from biological indicators are overstated.
Concerns Regarding Laboratory Error
The typical biological indicator used in steam sterilization of parts is spores of Geobacillus stearothermophilus. This thermophilic sporeformer grows best at 55–60°C and it would be a most unusual environmental isolate. Laboratory tests for biological indicators typically include both negative and positive controls. Terminal sterilization processes often utilize self-contained biological indicators that further reduce the risk of laboratory error and also improve test accuracy through the use of the same media as the Biological Indicator (BI) manufacturer and identical growth media in all test units.
Processing Mandates a Minimum Process
Demanding conformance to an arbitrary physical state without requiring microbiological evidence of lethality can increase patient risk.
Increasing awareness of the detrimental impact of overprocessing suggests a more flexible approach to sterilization cycles be utilized (17). The use of a standard cycle for all items without due consideration of the potential adverse impact on the finished product can add to patient risk. Terminal sterilization processes using lower temperatures and/or shorter times can provide added margins of safety over alternative aseptically filled products. Use of an appropriate biological indicator can support sterilization efficacy without the restrictions of the standard cycle.
Focusing on the BI Obscures the True Target
Microorganisms can survive without detection in what are otherwise perceived to be effective steam sterilization processes when they are present in locations where localized physical measurements either are not possible or are not representative of the process. The following examples taken from my personal experience outline some instances where physical measurements proved inadequate to predict spore death (18):
Spores embedded in the crystals of minimally soluble active pharmaceutical ingredient in a terminally sterilized injectable formulation.
Microorganisms present in non-wetted dispensing ports of terminally sterilized large volume parenteral (LVP) containers.
Microorganisms located in the interface of the elastomeric closures and the container surface.
Spores surviving on the surface of sterilizing grade filters.
Spores surviving in sealed bags of elastomeric closures.
Microorganisms present in a terminally sterilized injectable solution with a low percentage of unbound water.
These and other similar events I have encountered provide conclusive evidence that physical measurements are not definitive evidence of sterilization process lethality.
Summation
The physical conditions associated with effective sterilization (that is, overkill) are stated in the Ph. Eur. or PDA's Technical Report #1, Validation of Moist Heat Sterilization Processes: Cycle Design, Development, Qualification and Ongoing Control.
For this method of terminal sterilisation the reference conditions for aqueous preparations are heating at a minimum of 121°C for 15 min. (5)
A sterilization design approach where minimal information is required about the product bioburden. A worst-case bioburden assumption is used to determine the delivered lethality needed to achieve a PNSU of 10−6 on or in the items being sterilized. When using this approach, the qualification program must demonstrate that both the FBIO and FPHY are greater than 12 minutes (19).
The referenced documents, either indirectly or explicitly, include expectations for SAL and PNSU that are associated with microbial destruction. Thus, although it might seem that the physical conditions sufficiently describe a satisfactory sterilization cycle, the stated processes rely on unstated assumptions related to microbial destruction. The cited values only have validity because substantial microbiological evidence had been previously gathered to support their efficacy. Additionally, although it may appear that physical measurements are more definitive and precise, they are little more than convenient placeholders based upon an assumed PNSU and expected bioburden population/resistance. To claim that physical measurements should take precedence in sterilization validation is clearly unfounded when the origin of those physical measurements being utilized is biological destruction. Physical methods are an expedient means of assessing cycle performance, but they should never be considered definitive evidence.
As a young man, the author was a member of a competitive rifle team. In order to establish one's score the targets were retrieved and examined to confirm the results. Assumptions based upon rifle position, sight accuracy, trigger actuation, bullet weight, and speed could never replace the direct evidence obtained from examination of the target itself. Sterilization is best assessed in the same fashion.
Sterilization is a critical part of processes for essential therapies and its successful operation is vital to patients worldwide. Assuring the safety of sterile products requires that sterilization processes be appropriately developed, validated, and executed. Sterilization processes rely on equipment and systems that deliver conditions lethal to microorganisms. To ensure their reliability a combination of both physical and biological knowledge is essential. Of those, microbiological evidence must be recognized as definitive, with the temperature data understood as merely corroborative.
Note
This article expands upon earlier publications that addressed the relationship between microbiological and physical methods for the evaluation of steam sterilization effectiveness (20, 21). It was prepared because in the nearly two decades that have elapsed since the first publication, the misinformation embodied in the EMA's preference for physical measurements persists. With EMA Annex 1 currently under revision, the potential to correct this major flaw in its sterilization recommendations exists. The reader is encouraged to comment upon this and other aspects in the revision draft of Annex 1.
Conflict of Interest Declaration
The author declares that he has no competing interests.
Footnotes
↵1 The terms probability of a non-sterile unit (PNSU) and sterility assurance level (SAL) are not exact synonyms; however, the means for their estimation are identical.
↵2 Although it could be argued that the standard cycle assumes that the bioburden is less resistant and lower in the population than the common steam indicator Geobacillus stearothermophilus, none of these assumptions is explicitly stated. Additionally, it should be recognized that this sporeformer is neither truly representative of the native bioburden nor the most resistant microbial strain to steam sterilization.
↵3 Overestimation of the required lethality using physical measurements where the real death curve is concave downwards results in overprocessing, which should be avoided. Were the curve to be concave upwards, physical measurements would suggest a shorter cycle than required to eliminate the biological indicator, an undesirable outcome as well.
- © PDA, Inc. 2019