Abstract
Vial capping plays a critical role in the drug product manufacturing process owing to the complex interplay of several adjustable process steps. Seal quality and integrity and containment assurance are essential for parenteral pharmaceuticals, as the vial's content may be contaminated or, in the case of highly potent drugs (e.g., antibody drug conjugates), may bear a risk of contamination. The residual seal force (RSF) method can enable further insight in capping equipment settings independently of the container closure system (CCS) and their resulting seal quality.
The present study investigates the accuracy of the RSF method focusing on different force settings, RSF development over time, distance between capping plates and vial neck (roller-axis), time point of flip-off button removal, and internal and external vial pressure differences (flight simulation and vials closed under vacuum).
Results show that the forces used on an RSF tester should be kept low to minimize CCS deformation, and a period of stable RSF values after the initial decrease should be implemented between capping and RSF measurement to increase accuracy. Variations in the distance between the capping plates and vial neck (roller-axis) can result in incomplete crimps or visual defects of the seals. In addition, the time point of flip-off button removal as part of the sample preparation had no significant impact on RSF measurements. Finally, pressure differences between the vial interior and exterior had no significant impact on the RSF data.
LAY ABSTRACT: Vial capping plays a critical role in the drug product manufacturing process due to the complex interplay of several adjustable process steps. Seal quality, integrity, and containment are essential for parenteral pharmaceuticals, as the vial's content varies and may be contaminated, sensitive to stress, and/or highly potent (eg, antibody drug conjugates). The residual seal force (RSF) method can enable further insight in capping equipment settings independently of the container closure system and their resulting seal quality.
In this study, we determined RSF values by applying different force settings of the RSF tester and investigated the influence of sample preparation on the determination of RSF. Furthermore, the capping process parameter roller-axis was evaluated by RSF and visual inspection. In addition, we investigated the influence of pressure differences of vials on the RSF as they occurred during air transport and products closed under vacuum.
Introduction
A container closure system (CCS) for parenteral pharmaceuticals consists of three main components: a glass vial, a rubber stopper, and an aluminum crimp cap (1). To ensure the integrity of the system components with respect to dimensions, physical properties, specifications, and manufacturability cannot be selected individually but need to holistically fit together to ensure product stability and quality.
The capping process plays a central role in the sealing of the CCS (2) and in maintaining physical integrity of the CCS during storage. Manufacturing equipment variability and major process parameters such as capping plate plunger distance, rotation speed of the turntables, and capping precompression force (2) are critical to achieve seal quality and ultimately container closure integrity (CCI).
Molecule classes like the toxins of antibody-drug conjugates (ADC) pose a subnanomolar IC50, which results in a 100 to 1000 times higher potency than a traditional anticancer agent (3). ADCs, therefore, represent a new category of highly potent therapeutics, which possess novel challenges in the manufacturing process (4), handling (5), packaging, and specifically CCS. Furthermore, it has been shown that ADCs show higher susceptibility toward degradation like oxidation (6) or aggregation (7) than their monoclonal antibody (mAb) component alone. These circumstances require high seal quality and CCI to ensure product quality and containment of the highly potent substance to protect the environment or healthcare professionals handling the vials.
Even minor integrity failures lead to gas exchange or moisture or bacterial ingress. Ingress of gas, specifically oxygen, can result in oxidation of the biopharmaceutics (8) or drugs (9). In addition, gas exchange during reconstitution of freeze-dried products can lead to incomplete solvent injection owing to pressure loss in the partial vacuum in the vial. For freeze-dried products, moisture ingress can destroy the freeze-dried cake structure and, therefore, might destabilize the active ingredient (10) and/or its excipients. Finally, microbial ingress can lead to a contaminated parenteral product and can generate serious side effects when injected in patients (11).
Two major approaches to test primary packaging are the broadly used mCCI (microbial ingress container closure integrity) and pCCI (physical container closure integrity) methods. The mCCI analyzes the ingress of bacteria into the vial when incubated in a bacterial suspension. The pCCI analyzes the integrity of the CCS using physical or chemical methods. A direct correlation between mCCI and pCCI testing has been presented by Kirsch et al. (12) and Morrical et al. (13). Advantages and limitations of the most commonly used CCI methods are discussed in Kale et al. (14).
All manufactured vials are subject to visual inspection (manual and/or automated) to ensure consistent seal quality, whereas inappropriately sealed vials are rejected and removed from the batch. Seal quality can be tested by vial height measurement, computed tomography, and residual seal force (RSF) testing (15).
RSF allows a quantitative analysis of the capping equipment settings and the resulting seal quality of the CCS. One of RSF's main advantages is its format and capping equipment independency. In addition, the result of the RSF method is expected to be independent of the operator, which in turn generates a more consistent data set and more comparable results.
RSF values have been shown to vary depending on the rubber stopper compression level (16). Therefore, RSF provides the opportunity to judge the capping result and its quality. It has been observed that an increase of rubber plug compression decreases the risk of leaks (17). Consequently, RSF testing of the capping process parameters enables a first investigation of the seal quality and provides the possibility to optimize the capping equipment settings (2). The RSF value is determined by the compression stress applied by an anvil in comparison to the distance the anvil travels (18⇓–20). RSF values change with application of stress on the rubber plug as well as over time. This can be illustrated by applying the degenerative Maxwell curve (18). Change in the RSF values results from a stress relaxation of the elastomer. It is important to ensure CCS sealing not only during the manufacturing process but also throughout storage of the pharmaceutical product.
RSF also represents a seal quality parameter independent of the variation in dimension of the glass vial neck, rubber plug, and aluminum cap. This standardization would complement the currently practiced techniques including visual inspection, which is highly operator dependent and provides only a superficial insight in CCS seal tightness.
This study investigated conditions through which the RSF values are determined and which parameters impact the results. The study also addressed shelf-life and transportation conditions that could directly impact the RSF and, subsequently, the seal quality over time. The study focused on the following parameters and their impact on the RSF: the variation in distance between the capping plates and vial neck, RSF over time, the time point of the flip-off button removal, and the pressure differences between the vial interior and exterior.
Materials and Methods
Vials, Rubber Stoppers, and Crimp Caps
Type I glass vials (2 mL and 15 mL) were purchased from Schott (Mainz, Germany). The vials had a vial neck diameter of 13 mm (2 mL) and 20 mm (15 mL). Two different designs of the same rubber stopper elastomer formulation were used in this study: the Daikyo D777-1 serum rubber stopper as well as the corresponding freeze-drying type design from Daikyo Seiko (Tokyo, Japan). The different vial and rubber stopper combinations were capped with crimp caps from Dätwyler (Altdorf, Switzerland) and/or West (Exton, PA, USA). All materials were taken as received from the suppliers without further pretreatment (such as washing or sterilization). The same batch of vials, stoppers, and crimp caps were used for all experiments to exclude potential batch-to-batch variability.
Vial Capping
The different vial, rubber stopper, and crimp cap combinations were crimped using an Integra West Capper (Genesis Packaging Technologies, Exton, PA, USA), a lab-scale capping machine. Capping precompression force, capping plate-plunger distance (distance between plunger and capping plate), and roller-axis (distance that the capping blades/rollers are moving toward the vial neck) were modified during this study in order to investigate the resulting RSF values. Other capping parameters, such as capping plate shape and capping plate angle, were kept constant in all experiments.
Five different capping-force and capping plate-plunger distance combinations were applied to seal 2 mL and 15 mL vials (Table I). The 2 mL vials feature a smaller vial head and are equipped with smaller rubber stoppers compared to the 15 mL vials. Therefore, overall smaller capping plate–plunger distances and higher roller-axis distances were required for the 2 mL vials to achieve an acceptable capping result.
Overview of the Capping Equipment Settings
The capping plate–plunger distance can be adjusted in 0.025 mm steps; the capping precompression force can be set to 10 Ib and up to 60 Ib.
RSF Measurement
The automated RSF tester AWG from Genesis Packaging Technologies (Exton, PA, USA) was used. The concept of RSF determination is described in detail by Morton et al. (17, 18) and Ludwig et al. (19, 20). RSF is measured by applying compression at a fixed rate to the cap/stopper/vial combination and then collecting strain (distance) versus stress (force) data. Special cap anvils were produced to fit the Dätwyler and West crimp caps. The verbose mode was applied to collect the raw data for all measurements. The RSF tester allows the selection of four maximum force settings (111, 156, 200, and 245 N) to determine the RSF value. The standard maximum force setting of 111 N was used (unless mentioned otherwise). The measured sample groups contained 20 vials. The AWG RSF tester uses between three and five individual measurements, from which it extracts three consistent results and determines the average. The additional measurements are disregarded. The crimped vials were rested for a period of 24 h at room temperature prior to their testing (unless mentioned otherwise). The flip-off button was removed right before the RSF measurement (unless mentioned otherwise). Every vial was measured only once. It should be noted that all experiments have been conducted at room temperature.
Pressure Chamber
Lyo Vials:
The 15 mL vials with lyo rubber stoppers were closed with an inner vial pressure of 600 mbar using the LyoStar freeze dryer (SP Scientific, Gardiner, NY) and the DVR 5 (Vacuubrand) pressure detector. Vials were crimped using West caps and five crimp settings (Table I). Vials were measured after 24 h and after 1.5 months; no vial was measured twice. After the RSF measurement, the pressure within the vial was investigated using the Digital Pressure Indicator DPI 705 IS (GE) (New Fairfield, CT, USA).
Transport Simulation:
The 15 mL vials were crimped with serum or lyo rubber stoppers and West caps using the described five different crimp settings (Table I). The ASTN-D6653 standard was applied to simulate a realistic air transport. The precooled vials were placed in the LyoStar (FTS), and a pressure of 500 mbar was applied for 15 h at 5°C. The pressure level was additionally monitored by the DVR 5 (Vacuubrand). Then the vials were brought back to 1000 mbar and stored for another hour at 5°C. The vials were brought back to room temperature after the “landing” and another measurement was done. After the RSF measurement, the pressure within the vial was investigated using the Digital Pressure Indicator DPI 705 IS (GE).
Results and Discussion
Influence of the Applied Force Setting on the RSF Value
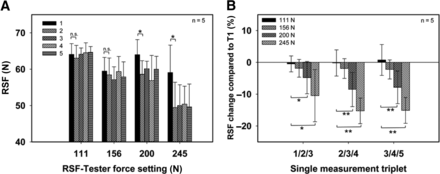
The influence of different force settings on the determined RSF value was examined. Vials using one set of container closure components and capped with one capping equipment setting (setting 4 in Table I) were analyzed for their RSF. All four possible force settings (111, 156, 200, and 245 N) of the RSF tester were applied to five different vials each and the determined values were compared (Figure 1).
Influence of the applied force setting on the RSF value.
(A) Five single measurements in a row. (B) Normalized change in RSF caused by averaging triplets. Error bars represent the standard deviation of values determined from five separate vials. Statistic is being represented by *p > 0.01 and **p > 0.005.
The usage of higher forces enabled the determination of higher residual seal forces and, therefore, could be applied to reveal a wide range of RSF values. Each vial was measured using five continuous and independent single measurements (Figure 1A). Vials measured using the force setting 111 N resulted in an RSF of 64.1 ± 3.0 N with no significant changes over the five single measurements, as did the measuring force of 156 N, which resulted in 59.5 ± 3.8 N.
The force setting 200 N showed a drop in RSF between the first and the second single measurement from 64.0 ± 4.1 N to 58.6 ± 3.8 N. This was also observed for the force setting 245 N, which showed a drop in RSF from 59.0 ± 7.5 N to 49.6 ± 6.9 N. The force settings 200 N and 245 N showed a significant drop (p > 0.005) in RSF between the first and the second single measurement.
This observation suggests that higher forces can lead to a loss in performance and quality of the crimped vials (possibly because of irreversible rubber stopper deformation). These changes can be observed in the RSF obtained by the RSF tester. These results suggest that the data created using the force settings of 200 N and 245 N should be handled carefully when using more than the first single measurement.
The current AWG RSF measuring concept includes a multi-data collection process. The RSF tester repeats the data collection process three to five times until three results close enough to each other are obtained according to the preprogrammed algorithm. The average of these three consistent results is then reported as RSF.
Figure 1B shows the measurements in Figure 1A as an average of single measurements 1–3/2–4 and 3–5 exemplarily. The values have been normalized and refer with 0% to the value determined by the first single measurement.
Data obtained using the same force conditions resulted in no significant changes throughout the average of three of the five single measurements as shown in Figure 1A. This was shown for all four force settings and, therefore, confirms the reproducible readout of stable and consistent data. Furthermore, no significant differences were observed between the average on force settings 111 N and 156 N. This indicates that data obtained using these two force settings can be directly compared.
In contrast, data obtained by using the force settings 200 N and 245 N resulted in a significant change (Figure 1B) compared to the force setting 111 N. The average of single measurements 1–3 differed between the force settings of 111 N and 200 N already around 4.8% ± 5.1% and further increased to 8.6% ± 5.4% for the average of single measurements 2–4 and 3–5. Differences between 111 N and 245 N were even greater with 10.5% ± 8.2% for the average of single measurements 1–3 and reached up to 15.2% ± 4.0% for the average of single measurements 2–4 and 3–5.
A direct comparison of the averaged data obtained with different force settings is, therefore, not given and should be avoided.
These findings suggest that data obtained using the same force setting can be directly compared. Furthermore, it was shown that multiple measurements of high-force settings can manipulate the RSF measurements owing to modification of the crimped CCS caused by the RSF measurement. Therefore, measuring a single vial multiple times is not advised for higher force settings. It also was shown that absolute values determined by the processed average of different force settings cannot be directly compared. The higher force settings should therefore be avoided for standard CCS configurations. If they are applied, using a single measurement (and a sufficiently high sample size) can potentially provide more exact and reliable data.
Development of RSF Over Time
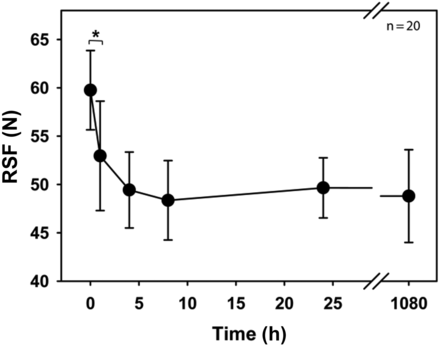
Investigations by Mathaes et al. (16) suggested a resting period between capping and the RSF measurement to achieve higher accuracy. The capping process causes compression of the rubber. These forces are at their maximum right after the vial capping. Over time, the rubber refocuses these forces to achieve a partial relaxation. This relaxation can cause changes in the RSF values. This study investigated the RSF development right after capping until one and a half months after capping (Figure 2).
Development of RSF over time.
West 20 mm crimp cap, D777 serum rubber stopper, 15 mL vial. Error bars represent the standard deviation of values determined from 20 separate vials crimped using setting 3. Statistic is being represented by *p > 0.005.
The RSF determined right after the crimping was the highest measured with 59.8 ± 4.1 N. Within the first hour, the RSF dropped significantly (p < 0.05) to 53.0 ± 5.7 N. The RSF continued to drop until about hour 4, at which the RSF seemed to flat-line at 49.4 ± 3.1 N, which remained stable over the next one and a half months (1080 h).
These results indicate that the time-period between capping and RSF measurement should be kept constant to achieve the best comparability between result sets. The RSF over time development could be primary packaging component-specific. Therefore, these data are only representative for the CCS tested, because a different vial format or rubber hardness could alter the RSF development.
However, the fact that RSF reached a baseline at about 4 h allows a direct comparison of result sets measured after a 4-hour resting period between capping and RSF measurement for this CCS.
Influence of Roller-Axis on RSF
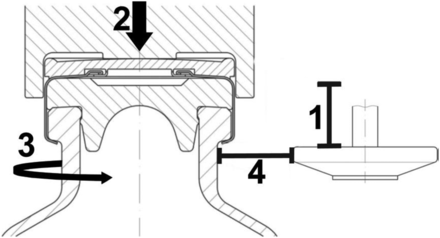
Capping is a mechanical process that can be influenced by multiple factors. Three capping parameters and their impact on RSF were investigated previously (2) (Figure 3): (a) capping plate–plunger distance, (b) precompression force, and (c) rotation speed of the turntable.
Schematic presentation of capping process parameters. 1 = Capping plate-plunger distance, 2 = precompression force, 3 = turntable rotation speed, and 4 = roller-axis.
This study evaluated a fourth parameter on the RSF: the roller-axis. This parameter describes the distance between rollers/capping plates and the vial neck as illustrated by distance #4 in Figure 3. This means the larger the roller-axis value, the smaller the capping plate–vial neck distance. The lab-scale Integra Westcapper (Genesis) allows an easy adjustment of the roller-axis, which can be varied over a relatively wide range with this specific equipment. The investigation included roller-axis values between 10.5 mm and 16.5 mm for two different types of crimp caps. The use of two different crimp caps with slightly different dimensions like the aluminum skirt length could result in different crimp qualities caused by the roller-axis setting.
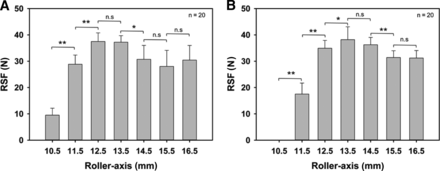
The roller-axis distance showed a clear impact on the RSF for both crimp cap types (Figure 4, A and B). The capping process showed an incomplete crimp with low or non-measurable RSF depending on the cap type, when the roller-axis value is too low and, therefore, the distance between capping plate and vial neck is too large.
Influence of roller-axis on RSF.
(A) Dätwyler and (B) West 20 mm crimp cap, D777 serum rubber stopper, 15 mL vial. Vials were crimped using setting 3. Error bars represent the standard deviation of values determined from 20 separate vials. Statistic is being represented by *p > 0.01 and **p > 0.005.
The Dätwyler cap showed an RSF maximum of 37.5 ± 3.3 N at the 12.5 mm to 13.5 mm roller-axis settings, which dropped significantly by further increasing the roller-axis setting. Visual defects like waves in the aluminum were observed starting at a 14.5 mm roller-axis setting. The West cap showed an RSF maximum of 38.2 ± 4.9 N at the roller-axis settings of 13.5 to 14.5 mm, which also dropped by further increasing the roller-axis setting.
The RSF readout agreed with the visual inspection in terms of an incomplete crimp with high roller–vial neck distances, which showed a low RSF value and an increase in RSF by shortening the capping plate–vial neck distance. Optimal capping equipment settings were identified using the RSF measurement in combination with a visual inspection.
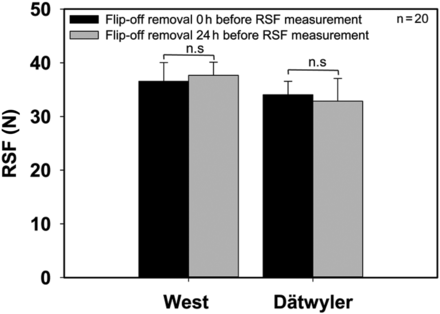
Influence of Flip-Off Button Removal Time Point on RSF
Sample preparation for the RSF measurement involves the removal of the flip-off disc. Mathaes et al. (21) showed that removal of the flip-off disc decreased the standard deviation and simplified the pressure-versus-distance profile, which allows the algorithm of the AWG RSF tester to determine a more exact RSF value.
Two different flip-off button types were tested to determine if the flip-off button removal time point impacted RSF (Figure 5). The West crimp cap has pre-cut aluminum bridges to connect the flip-off with the aluminum coat, and the second type from Dätwyler has “fingers” to connect the flip-off with the aluminum. The removal of the flip-off button represents physical stress on the aluminum cap and the elastomer, which comes with the potential risk of influencing the RSF by deforming or damaging these components. Operator subjectivity is involved in the measurement process by removing the flip-off with different strength or technique. This gives reason to consider further whether a device to remove the flip-off consistently should be used to eliminate operator subjectivity.
Influence of flip-off button removal time point on RSF. Dätwyler or West 20 mm crimp cap, D777 serum rubber stopper, 15 mL vial.
Vials were crimped using setting 3. Error bars represent the standard deviation of values determined from 20 separate vials.
Two time points were chosen to remove the flip-off buttons. The flip-off buttons of the first set of samples were removed just before RSF measurement. The flip-off buttons of the second set of samples were removed right after finishing the crimp process and were then stored for 24 h prior to their testing. Both sample sets were measured after 24 h storage at room temperature.
If the flip-off button removal impacted the RSF, a difference between the two time points would have been expected. A deformation of the elastomer or aluminum cap would temporarily influence the RSF value. For example, a further compression of the CCS could lead to a further increase of the RSF. This rise in RSF would decrease over time. Such a change would be detectable in this study design. Both sample sets showed no significant differences in RSF at both time points and, therefore, showed no impact on the RSF. This suggests that a flip-off button removal device is not necessary to further stabilize the RSF measuring process.
Influence of Pressure on RSF
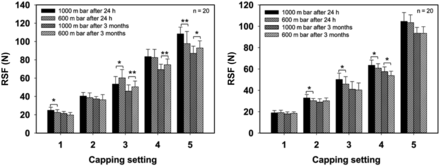
Scenario 1—The Interior Vial Pressure is Lower Than the Exterior Vial Pressure:
All studies performed to this point have been executed with no pressure differences between the vial interior and the vial exterior. However, freeze-dried products are closed under vacuum, which allows an easier reconstitution and better handling and enables a CCI measurement by determining the inner vial pressure. So far, it has not been investigated if such a pressure difference causes a short- or long-term impact on the RSF. This study included an investigation of 2 mL and 15 mL vials between the time period of 24 h and 3 months (Figure 6). A potential risk is that the low inner vial pressure exerts a pulling force (and as an extreme could suck the rubber stopper partially into the vial), which would decrease the tension within the elastomer and its compression, which would then result in a decrease of the RSF.
Influence of inner vial pressure on RSF.
(A) West 13 mm crimp cap, D777 lyo rubber stopper, 2 mL vial and (B) West 20 mm crimp cap, D777 lyo rubber stopper, 15 mL vial. Error bars represent the standard deviation of values determined from 20 separate vials. Statistic is being represented by *p > 0.01 and **p > 0.005.
It was observed that independent of the inner vial pressure, the RSF of all five capping settings and both CCS configurations decreased over the period of 3 months compared to the measurement after a single day. This was more dominantly detectable for vials having a high RSF. The inner vial pressure was confirmed after the RSF measurement (data not shown) using a digital pressure indicator DPI 705 IS (GE) to exclude the risk of pressure adoption through leaks.
Large and small vials showed no trends with the inner vial pressure differences independent of the capping setting and storage time. Minor changes in RSF, but not significant trends, were observed, which identify a low inner vial pressure as a risk. However, these differences in RSF are not large enough to impact seal quality. The drop over the 3-month period could be a result of changes in rubber stopper moisture, which result in alterations of the physical properties of the rubber (22).
Results indicate that an inner vial pressure as low as 600 mbar has no significant impact on the change of RSF over time independent of the capping setting or vial format. This would allow the application of the RSF measurement throughout the shelf-life of lyophilized products to evaluate seal quality.
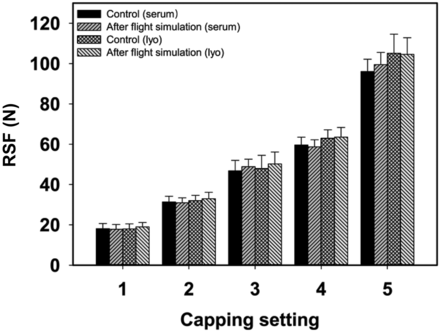
Scenario 2—The Interior Vial Pressure is Higher Than the Exterior Vial Pressure:
Transportation is part of the life cycle of every vial. Transportation could be conducted by truck, train, ship, or plane. The quality of the seal must be ensured for all modes and steps of transportation. Transportation by truck, train, or ship mainly has the physical stress of shaking, but air transport brings an additional challenge, which is the pressure difference during the flight. In contrast to scenario 1, where the inner vial pressure is lower than the outer vial pressure, air transport introduces the opposite phenomenon, because the inner vial pressure is higher than the outer vial pressure (inner vial pressure > outer vial pressure). The potential risk is that the rubber stopper could be pushed upward against the aluminum cap during the air transport, which could cause a deformation of the aluminum cap. This deformation of the aluminum cap could cause relaxation of the elastomer and, therefore, a long-term drop of RSF, which represents a CCI risk. A flight simulation was executed according to the ASTN-D6653 guideline. The air transport simulation was performed at 5°C, and a vacuum of 500 mbar was applied for 15 h to ensure a realistic air transport. Vials were closed with both serum and lyo rubber stoppers with an inner vial pressure of 1000 mbar to create a worst-case scenario.
The RSF results of both CCS configurations and all five capping equipment settings showed no significant changes with or without air transport simulation (Figure 7). This suggests that air transport of crimped vials does not significantly impact the RSF and, therefore, does not indicate changes in seal quality. Furthermore, these results indicate that the RSF methodology would be applicable to determine seal quality throughout the shelf-life of crimped vials.
Influence of air transport simulation on RSF. West 20 mm crimp cap, D777 serum or lyo rubber stopper, 15 mL vial.
Error bars represent the standard deviation of values determined from 20 separate vials.
Conclusion
CCS seal quality is highly important for parenteral drugs, and a respective analysis could be promising for highly potent and stress-sensitive substances like ADCs. RSF is a powerful method for determining the rubber stopper compression and, therefore, tightness of the seal and nicely complements CCI methods like helium leak testing. The forces used on an RSF tester should be kept low to minimize deformation of the CCS, and a period of sufficiently stable RSF values after the initial decrease should be implemented between capping and RSF measurement to increase accuracy. One additional capping process parameter (roller-axis) was introduced, and its effect on RSF was investigated. The time point of the flip-off button removal did not influence RSF. Finally, the RSF method did not reveal any impact of interior and exterior pressure differences, which qualifies the RSF methodology to evaluate crimp quality throughout the shelf-life of a vial.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgment
We specially thank Holger Roehl who provided access to essential equipment to conduct this investigation.
- © PDA, Inc. 2019