Abstract
In practice it is claimed that load characteristics influence a surface steam sterilization process. To the best of our knowledge, no information on this topic has been found in the literature. The purpose of this study was to find if a load influences the duration and related characteristics of a surface steam sterilization process. In a case study, every load monitoring using an objective, quantifying steam penetration test was performed during 30 days. This resulted in 98 production processes with load monitoring. The acquired data were analyzed. A relation between the weight of a load and the duration of a surface steam sterilization process has been found. In this case study, it is demonstrated that the process time increases with the weight of the sterilizer load. Additionally, it is concluded that when the duration of a process is longer, diffusion will have a greater effect and steam penetration increases.
LAY ABSTRACT: Steam sterilization is applied in most health-care facilities that reprocess medical devices, in pharmaceutical industries, and in labs where specific instrumentation and equipment have to be sterile. Steam sterilization is still not fully understood, as demonstrated in the literature. Our manuscript contributes to understanding the surface steam sterilization process. The information shared in our manuscript demonstrates by a case study that relations exist between the weight of the load which is steam sterilized, the duration of a process, and the steam penetration in a process. A quantitative relation can be used to predict the duration of a process and the steam penetration. It is likely that for other steam sterilizers these relations can be found as well. This is of interest for institutes researching decontamination and sterilization, health-care facilities, developers and manufacturers of medical devices, and committees addressing standards for steam sterilization.
Introduction
Steam sterilization is not yet fully understood (1, 2). Still, the most applied method in hospitals for sterilization of reusable medical devices is surface steam sterilization. In pharmaceutical industries and laboratories, surface steam sterilization is also applied (3). More information on the subject would make this technology more evidence-based. Surface steam sterilization conditions are specified in the literature, that is, saturated steam at a predetermined time-temperature combination (4). The standards for steam sterilization suggest similar conditions (5⇓–7). These conditions should be established in every sterilization process on all surfaces that are in contact with the environment, including inner surfaces of instruments with channels. Recent studies indicate that these sterilization conditions are not always established on all surfaces in each production process (8). The sterilization conditions depend on the process time. In the literature, quantitative relations between the weight of the load and the process time are reported (3, 9), but no qualitative explanation or relation has been found for the steam penetration capacities of processes. It would be helpful to find the causes of the observed variations.
The aim of (steam) sterilization is to make items free of viable organisms (10). This cannot be measured directly on a sterilized item before its use, because once tested (or used), the device is not sterile anymore. Therefore, validation and monitoring procedures are developed to ensure efficacy and reproducibility of sterilization processes (7). These procedures are based on microbiological kill and tests (4, 7, 11). In all sterilization methods, the sterilization agent must be in contact with the viable organism for it to be killed. For surface steam sterilization, this implies that steam has to penetrate to all surfaces to which the patient has access to or is exposed to, where medicines are produced, or where tests are conducted. For example, steam should reach the inner surfaces of devices such as hoses in pharmaceutical industry, glass instruments in labs, and channeled medical devices. The standards specify steam penetration tests (12) or air removal tests (13) to ensure steam sterilization conditions on the inner surfaces of devices. The result of these tests is a “pass” or a “fail.” A pass result means that the process has sufficient steam penetration or air removal to produce sterile loads. A fail result indicates that the process does not have sufficient steam penetration or air removal and measures should be taken before using the sterilizer for production.
Daily, before starting production with a steam sterilizer, a steam penetration test (SPT) has to be performed (5, 7). The rationale behind this daily test is that the steam penetration capacities of a process in that particular sterilizer are checked before starting production. According to the standards (6), the process used for the SPT should have the same “conditioning phase” (Figure 1) as the production process. Obviously, the standards assume that when the SPT result meets the requirements, the corresponding production process will achieve sterilization conditions in the load as well. Consequently, these requirements for an SPT (12) specify the steam penetration capacities of a steam sterilization process, as mentioned above.
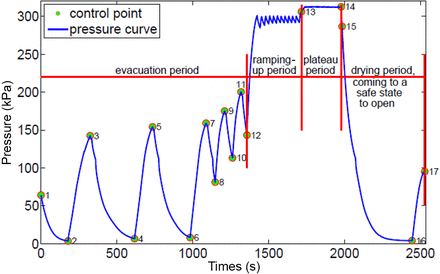
The Miele process installed on sterilizer 2 of the Catharina Hospital. The control points of the process (CPs) are numbered. The period between CP 1 and CP 13 is often called the “conditioning period”. Here this period is divided into the “evacuation period” between CP 1 and CP 12, where the initially present air is removed out of the chamber, and the “ramping-up period” between CP 12 and CP 13. In this period, the load is brought to an elevated temperature to enter the “plateau period,” the period between CP 13 and CP 14. According to the standards (6), this period consists of the “equilibration” and “holding time.” In the period between CP 14 and CP 17, the load is dried and the sterilizer is brought to a safe state to open (the pressure in the sterilizer chamber is close to or equal to the environmental pressure).
Although the test is not a direct microbiological method, it is meant to check if sufficient steam penetration is established to claim sterilization conditions. Using this criterion (12) for steam sterilization conditions, an every load monitoring (ELM) method is applied. The present case study demonstrates the relation between the weight of the load and the duration of a steam sterilization process and, consequently, the steam penetration capacity of that process.
Method and Materials
In the period from August 30 to October 27, 2016, ELM was performed on one sterilizer during 30 days. The ELM was performed according to a protocol. The protocol specified that a 3M™ ETS (electronic test system; model 4208, 3M™, Germany) should be used. The performance requirements defined for alternative SPTs in the standard ISO 11140 part 4 (12) were used as criteria. The SPTs were performed to be in compliance with the standards (6, 7) for a normal production day and during the data acquisition.
The criteria applied here are specified in the standard ISO 11140 part 4 (12). These criteria were chosen because the sterilizer is situated in Europe (in the Central Sterile Services Department (CSSD), Catharina Hospital, Eindhoven, The Netherlands). In countries were the European Standards are used ISO 11140 part 4 (12) should be used for the SPT, whereas in other regions other requirements could be applicable, for example, ISO 11140 part 5 (13).
A 3M™ ETS was specified because during the data acquisition period, it was the only commercially available SPT that can quantify the steam penetration with a numerical value (14). Figure 2 shows an ETS device. The functioning of this device is described in the literature (14, 15). Because the ETS is calibrated to a textile towel pack (6, 12), it represents a textile pack and can be used as an SPT (6, 12). The construction of the ETS (14, 15) may represent a channeled device with a thick wall, as well. Following the Instructions for Use, the ETS was used unwrapped, without microbiological barrier. A microbiological barrier is used when medical devices are sterilized to protect them from recontamination.
Sterilizer 2 (Miele model PS 5662) located in the CSSD of the Catharina Hospital was used. The sterilizer was validated and its compliance with the applicable standards (6, 7) was ensured. Figure 1 shows the sterilization production process (SPP) at a sterilizer temperature of 134 °C. The SPT process has an identical structure, but the “plateau period” and the “drying period” are programmed to be shorter than those in the SPP. The protocol to acquire the data was developed together with the operators of the sterilizer. It basically contained the following steps:
For every load of the sterilizer a form (Form A: ETS in ELM) had to be filled out. Amongst others, the following aspects had to be registered: the operator, the ETS used, a detailed description of the load, the tray, the number of trays, the contents, the type of load and medical devices (hollow, solid, metal, plastics and porous) and the wrapping method.
The load consists of trays. Each individual tray had to be composed according to a protocol from the CSSD. The protocol specified the trays to be used, the devices to place in the tray, and the methods of how to wrap the tray.
Every load consisted of surgical instruments. (For traceability reasons, the instruments are registered via an automated system and the protocol of the hospital.)
A complete load for one process was composed according to the protocol of the CSSD.
After the load was identified, the weight of the load had to be registered.
During the loading of the sterilizer not only the positioning of the load but also the position of the ETS had to be registered.
Before starting the process, it had to be checked if the ETS was ready for use (switched on) and located at the registered position in the load.
The sterilization process was started.
After the process was finished, the load was taken out of the sterilizer.
The ETS was taken out of the load.
The data acquired with the ETS was downloaded to the ETS software (3M (model 4110, 3M).
To link the load and the ETS file, the name of the ETS file was registered on Form A.
Form A and the data acquired with the ETS were archived systematically for the data analyses.
The ETS complies with the performance requirements of the standard for steam penetration (12). These requirements are based on a defined towel pack that originates from the Bowie and Dick (BD) autoclave tape test (16). It means that when the defined towel test pack for steam penetration would show a fail result in the specified processes for steam penetration testing (12), the ETS will show a fail result as well. On the other hand, when the defined towel test pack would show a pass result in the specified processes (12), the ETS will show a pass result as well. These characteristics for testing are captured in an algorithm of the ETS and are “translated” into a calculated BD-value. When the BD-value ≤0, the steam penetration is insufficient, and the ETS will show a fail result. When the BD-value is >0, the steam penetration is sufficient and the ETS will show a pass result. The higher the BD value, the better the steam penetration of a process. The ETS calculates the BD-value from the start of the process to the end of the “plateau period”, CP 1 and CP 14 in Figure 1, respectively. Because the ETSs are calibrated individually, they are interchangeable.
Before using the ETS, its core temperature has to be lower than 35°C. If the temperature is higher at the start of a process, the ETS will show a “no result.” This means that the BD-value is not valid. The ETS is an electronic device and has to be ‘switched on’ before use in a steam sterilizer. If the ETS does not recognize a process within 10 min after being switched on, it falls back into a “sleep mode.” In the sleep mode, the ETS will switch itself on automatically during the “evacuation period” of the process. Because not all data of the evacuation period are registered and thus available to calculate the BD-value, the ETS will show a no result. These situations should be avoided but can be caused by human error.
Results
During the 30 days of ELM, data sets consisting of 31 SPTs according to the existing standards (12) and 98 SPPs with load were acquired. There were 31 SPTs instead of 30 because on one day, the SPT indicated a fail result. The second sequential SPT on that day indicated a pass result, meaning that the sterilizer is ready for production according to the standards (7).
In all SPPs, the ETS was positioned at the same location, in the middle of the lowest loading level. Two of the 98 SPPs had insufficient steam penetration according to the standard ISO 11140 part 4 (12). In three cycles, the weight of the load was not registered precisely enough. In three measurements, the ETS was too hot to calculate a valid BD-value, and in three processes, an SPP was not started correctly. In two SPPs, the “plateau period” was shorter than 240 s, the standard plateau period of the SPP. Possibly an SPT process was started instead of a standard SPP. These 13 processes were not used in the further data analyses. By excluding these results, the data sets include only files of processes that meet at least the minimum requirements for steam penetration (12). This yields 87 SPP (with load) data sets for the data analyses. In Table I, the BD-value and duration of the plateau period of these SPPs are presented. The measurements with the ETS yielded a mean duration of 261 s.
BD-Values and “Plateau Period” Duration of the SPP with Load for ELMa
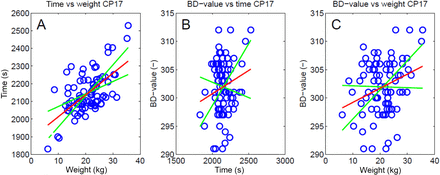
In Figure 3, the BD-value, weight of the load, and time are plotted against each other. The first-order polynomials (y = ax + b) or trend lines can be approximated using the following equation:
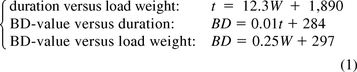 in which W denotes the weight (kg), t the time (s), and BD the BD-value (−). Also, the slopes of the 3σ-curves (99.7% probability limits) are presented in this figure. A closer inspection of eq 1 set reveals that both the BD-value and the duration (time) of a process depend on the weight of the load: the greater the weight, the more energy is needed to heat up the load and, consequently, the longer it takes to heat up the load. The 3σ-boundaries in Figure 3A indicate that the duration of a process increases with increasing weight. Also, the boundaries in Figure 3, B and C, indicate that the BD-value increases with increasing time and weight, but these dependencies are not very pronounced. This made it interesting to further investigate the relation between weight and time for the different process periods (Figure 1) and to find in what period weight has the largest effect.
in which W denotes the weight (kg), t the time (s), and BD the BD-value (−). Also, the slopes of the 3σ-curves (99.7% probability limits) are presented in this figure. A closer inspection of eq 1 set reveals that both the BD-value and the duration (time) of a process depend on the weight of the load: the greater the weight, the more energy is needed to heat up the load and, consequently, the longer it takes to heat up the load. The 3σ-boundaries in Figure 3A indicate that the duration of a process increases with increasing weight. Also, the boundaries in Figure 3, B and C, indicate that the BD-value increases with increasing time and weight, but these dependencies are not very pronounced. This made it interesting to further investigate the relation between weight and time for the different process periods (Figure 1) and to find in what period weight has the largest effect.
Duration of the process plotted against the weight of the load (A) and the BD-value plotted against the duration of the process (B) and plotted against the weight of the load (C). The red lines are first-order polynomials (trend lines) of the data points. The green lines represent the slope of the 3σ-boundaries (99.7% probability limits). The intercepts of these lines with the red lines are adjusted to the mean of the values for the sake of clarity.
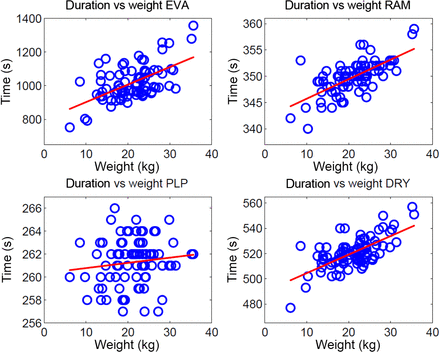
Table II and Figure 4 present the duration of the different periods of the SPP (Figure 1). A closer inspection of the table shows that the variation of the total SPP duration is 27.6%. The largest contribution to this variation is the evacuation period, with 44.6% variation. The duration of the plateau period is programmed in the sterilizer controller. Therefore, the variations of the plateau period (0.8%) are relatively small or even negligible compared to the other periods.
Duration of the Fastest and Slowest SPPs and their Process Periods (Figure 1)
Time duration of various process periods of a sterilization process against the weight of the load. EVA stands for “evacuation period”, RAM for “ramping-up period”, PLP for “plateau period,” and DRY for “drying period.” The red lines are first order polynomials (trend lines) of the data points.
With a first-order polynomial, the trend lines of the different periods are calculated:
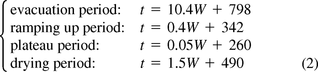
The eq 2 set indicates that the duration of all four periods increases with increasing weight. The evacuation period has the steepest trend line, indicating that in this period, weight has the largest effect (as is also reflected in Table II).
Similar analyses were performed for the other registered load characteristics, such as non-hollow (massive), hollow, and types of material. In these analyses (not presented here), no significant correlations were found. This made a detailed description of the load unnecessary.
Discussion
The standard (7) states that, “If the sterilization process relies on the removal of air from the sterilizer chamber in order to achieve rapid and even penetration of steam into the sterilizer load, a SPT shall be carried out each day before the sterilizer is used”. In practice this is often translated as “Perform a Bowie and Dick–like test” (12, 13) before starting production. However, the literature indicates that in 1%-3% (8, 17) of the steam sterilization processes the steam penetration requirements (12,13) are not met. Consequently, when a sterilization process relies on the removal of air from the sterilizer chamber, this air removal or steam penetration should preferably be checked in each load. Because the aim of sterilization is to deliver products free of viable organisms, the criteria for steam sterilization are based on biological kill. In European CSSD departments, sterilization cycles are released on the basis of physical parameters. The criteria that are used to justify parametric release are comparable to Annex 17 of the GMP, for example, known bio-burden of the sterilized products, controlled decontamination process, validated sterilization process, and daily check of critical sterilization parameters like steam penetration and air leakage. In this case, it is not necessary to sterilize biological indicators with each batch.
The results of the case study reported here demonstrate that the duration and the BD-value of a sterilization process increase with the weight of the load. In this case study, the criteria for steam sterilization tests (12) were used as criteria to judge if steam sterilization conditions were satisfied. Thirteen (13%) of the 98 performed SPPs did not show a satisfactory (pass) result. This 13% arises from 11 (11%) human errors, which are specified in the previous section, and two (2%) tests which did not meet the performance requirements for steam penetration tests (12). The two tests that failed (2%) are on the same order as the results reported in a previous case study (8).
Effects of variables other than the weight of the load, such as characteristics of the load, like being hollow or non-hollow, type of material, or wrapping method, were not observed. This might be caused by the fact that most sterilized loads were a mix of the different defined categories of loads. However, it is more likely that the weight of the load is the dominant factor for the duration of the steam sterilization process. The results given by eq 1 and Figure 3 demonstrate that the duration of an SPP and the BD-value increases with the increasing weight of the load. This can be explained by the fact that the heavier the load is, the more energy is needed to heat it up. The energy to heat up the load is provided by steam. The more steam that is needed, the more time is needed to supply the steam. On the other hand, the vacuum control points, for example, CP 2, CP 4, and CP 6, are also controlled by pressure. Because a heavier load needs more steam, more condensate will be formed. The removal of the condensate during de-pressurization may also add time before reaching a control point.
An important effect of the increase of the time duration is that diffusion is more effective. The working mechanism and influence of diffusion of non-condensable gases and steam in steam sterilization processes have been reported in the literature (15, 18, 19). The standardized steam penetration (12) and air removal tests (13) are based on textile packs. These packs are built up from textile, a porous material. The diffusion mechanisms for porous materials and for channels are different. Relations between porous textile packs and hollow devices have been reported (14). The literature (15, 18, 19) indicates that with more time for diffusion, steam penetration in channels will be better. The ETS makes use of a challenge channel (tube) (15). Therefore, more time for diffusion will result in a higher BD-value, as demonstrated in eqs 1 and 2 and Figure 3. It has to be remarked that these results also indicate that there is a lower limit for the time duration of the steam sterilization process: a process cannot be shortened below a minimum time. This minimum time is dictated by the load and its characteristics.
The duration of the plateau period programmed in the controller of the sterilizer is 240 s for the SPPs. The average value according to the ETS data is 261 s. This time difference occurs because the ETS calculates the plateau period as the time that the chamber temperature is above 134°C. The sterilizer controller has a sterilization set point of approximately 135°C to ensure that the chamber temperature will not drop below 134°C during the holding time—the actual sterilization period (6). The time during which the temperature in the sterilizer is higher than 135°C is obviously shorter than the time during which temperature is above 134°C. The difference between the longest and the shortest plateau period is on the order of 2 s (Table II). This small time difference demonstrates that the process is reproducible for the sterilisation time.
The variation in the duration of the drying period (13.4%; Table II) can also be explained by the weight of the load. The greater the load present, the more steam is needed and the more condensate is formed. In the drying period, this condensate has to be removed. Often this means that the condensate has to evaporate. The more condensate that is present the longer this will take.
To avoid wet loads after ending a process, it is important to remove the condensate as much as possible during the process. In the conditioning phase and after the end of the plateau period, this can be done via the drain. The condensate that is left in or on a load needs energy to evaporate, for example, during the drying phase. To heat up a load, steam condenses on and in the load. Part of this condensate may drip off the load before the drying phase. The amount of energy in the load is therefore larger than or equal to the amount of energy that is needed to evaporate the remaining condensate. When this energy is transferred fast enough to the condensate, it will evaporate completely. After evaporation, the water vapor can be sucked out of the chamber via the drain during the drying phase. If the transfer of energy to the condensate is too slow, it cannot be removed via the drain, which will result in wet loads.
The penetration of steam in thick-walled and thin-walled channeled devices in steam sterilization processes is reported in the literature (18, 19). It is demonstrated that the orientation of thick-walled channeled devices is a decisive factor. It is also explained that the condensate formed in a thick-walled channel can block the channel completely (18). The complete blocking of a channel prohibits or stops further steam penetration. By choosing the orientation such that the formed condensate is drained out of the channels, the channels are not blocked and steam penetration is possible (18). If a channel is thin-walled, the heat going through the wall of the channel heats up the inner surface. For the usual sterilization processes, inner surface condensation cannot take place and the channel will not be blocked (19).
The position of the ETS was similar in all processes, in the middle of the lowest loading level. This position was chosen because this is the location which is normally used for the daily SPT. This position is kept in all processes to make the results comparable. Possibly, the location of the SPT may influence the result, for example, in the case that the injected steam would not “mix” with the other gases (air) present in the chamber. No literature on this topic has been found by the authors.
The criteria for sterilization conditions and steam penetration were developed more than five decades ago (4, 11, 16). Since then, these criteria have not been modified. However, steam sterilization equipment and surgical instruments have been modernized. Microbiologists or others might consider re-performing the “old” experiments with biological material and modern steam sterilization equipment.
Overall it can be concluded that this case study demonstrates that the duration of a steam sterilization process increases with increasing weight of the load. Only processes in which the criteria for steam penetration tests are satisfied (12) and sterilization conditions can be assumed were considered. Because of the longer time of the conditioning phase for heavy loads, diffusion has more time, resulting in better steam penetration.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgments
The authors thank the staff of the Central Sterile Supply Department of the Catharina Hospital (Eindhoven, the Netherlands) for their constructive cooperation and practical input in specifying the data-acquisition protocol and acquiring the data for this study.
- © PDA, Inc. 2019