Abstract
Sensitivity of drugs to one or more elements of the primary packaging is a serious concern for the pharmaceutical industry. Biologics in particular are highly sensitive, leading to a higher risk of incompatibility and stability test failure as worst-case scenario.
This potential incompatibility—and the consequent formulation instability due to the interactions between the drug and the primary container surface—may have multiple causes: the intrinsic nature of the container surface, leachables coming from the materials used, substances coming from the production process, or silicone oil droplets or other particles.
The Alba primary packaging platform was designed to have the same interface between the drug and the glass container surface on the different primary packaging containers in order to minimize the emergence of instabilities at later stages of formulation development. Alba containers are internally treated with an innovative cross-linked coating based on silicone oil lubricant, and the additional rubber components have been selected to minimize the possible differences between the container typologies.
This paper shows in great detail the reduction of the inorganic extractables released and the comparability of the performances of the different containers obtained using Alba technology.
The improvement has been demonstrated by stressing the containers with different extract solutions; Alba-coated containers show a strong reduction of inorganic extractables and of corrosion degree compared to spray-on siliconized and bulk products. The containers included in the Alba platform present comparable results, and this represents a strong advantage during the drug formulation development by facilitating the transition from one container to another.
LAY ABSTRACT: The sensitivity of drugs to one or more elements of the primary packaging is a serious concern for the pharmaceutical industry. Biologics in particular are highly sensitive, leading to a higher risk of incompatibility and stability test failure worst-case scenario.
This potential incompatibility—and the consequent formulation instability due to the interactions between the drug and the primary container surface—may have multiple causes: the intrinsic nature of the container surface, leachables coming from the materials used, substances coming from the production process, or silicone oil droplets or other particles.
The Alba primary packaging platform was designed to minimize these problems associated with the interaction between the drug and its primary packaging. This paper shows in great detail and with robust data the inorganic extractables release reduction and the delamination risk mitigation obtained using the Alba technology.
- Primary packaging
- Inorganic extractables
- Pharmaceutical glass containers
- Prefilled syringes
- Protein formulation
- Glass delamination
Introduction
The materials used for pharmaceutical primary packaging are scrupulously selected to be compatible with the contained drugs. Compatibility means that the inevitable interactions between the drug formulation and the container (e.g., adsorption of the drug to the container surfaces, elements leaching from the container into the formulation) are down to acceptable levels (1). Drug–container interactions can compromise the long-term functionality of the packaging and the chemical stability of the formulation. Indeed, the elements leaching into the drug formulation can adversely affect the appearance, the quality, and the efficacy of the medicaments and even cause health risks (1⇓⇓–4). In general, liquid drug formulations, unlike lyophilized products, have a higher tendency to interact with the primary packaging because of the stabilizer present in the solution and the surface energies involved (2).
In particular, in parenteral drugs, the presence of impurities that can be injected straight into the patient's body increases the level of safety concerns. As a matter of fact, the “degree of concern associated with the route of administration” for injectables was classified “highest” (5). It is important to evaluate the container suitability through the characterization of leachables and extractables. The leachables are elements found in the drug that originated from the manufacturing equipment during drug production and administration or that spontaneously leached during storage because of the prolonged contact with the primary packaging. In order to predict the presence of these elements, the preliminary characterization is focused on the extractables, the chemicals that are forcefully detached from the materials that will be in contact with the drug. The study is performed by treating the container under stressing conditions, usually represented by high pressure/temperature or by filling the container with an aggressive extracting solution, to accelerate this specific phenomenon. It is also possible to simulate aging with extracting media similar to the drug formulation (5). The idea behind the use of such challenging extraction methods is to obtain a list of elements, the extractables, which should provide a complete overview of the potential leachables. Even though there is no guarantee that the leachables are a subset of the extractables (3, 6)—as in the case of leachables that are adducts between extractables and product components—an extractables list provides precious information that obviously depends on the type of primary packaging material, e.g., plastic or glass (4, 6). Leachables studies, on the other hand, are challenging owing to the low concentrations involved and the matrix effects caused by the drug solution, which is composed of excipients, additives, and proteins (7).
Borosilicate glass containers—neglecting the possible rubber closure components—have a known list of extractables, as the glass has a specific and known composition; therefore, the real variable is the quantity of each element that can be leached into a specific drug solution (8). Nevertheless, the extractables determination can be used as predictive methodology for drug–container interaction issues. For example, a high concentration of specific elements in solution can be connected with the glass corrosion and delamination phenomena.
The second effect that can affect drug stability is delamination. This complex phenomenon depends on several factors: the intrinsic nature of the glass itself (composition, expansion coefficient, glass type), the conversion process of the glass tube into the container, and the drug solution in contact with the primary packaging (pH, buffers, additives) (9). After a prolonged period of contact with the pharmaceutical preparation, the delamination appears as glass flakes detached from the inner surface of the container and subsequently shed directly into the drug solution. The glass lamellae are usually flat, shimmering, thin flake particles that can be visible to the unaided eye. It is important to assess the delamination propensity of a glass container batch that is used for filling operations as demonstrated by recent recall cases (10) and several previous studies (9, 11⇓⇓⇓–15).
One way to reduce the risks of incompatibility between the drug and the container is to limit the amount of extractables and the delamination issue, both claims of the newly developed Alba platform. The Alba containers are characterized by an innovative inner layer, a novel cross-linked coating based on silicone oil lubricant. The Alba coating was developed to realize a platform of high-quality glass containers, all with comparable characteristics: reduced sub-visible particles release, improved injection performances (in case of syringe and cartridge formats), reduced inorganic extractables, and improved delamination propensity from the barrier effect of the layer. In fact, during the development of a new drug, the vial could be used for optimizing the first stage, while the subsequent steps would require the final primary packaging, like a pre-fillable syringe for injection. The Alba platform minimizes the risk of incompatibility between the drug and the container, reducing the time-to-market with a common drug–container interface among all the formats and an accurate rubber components selection (16).
This paper is the second in a series dedicated to the Alba containers. In the first publication (17), the sub-visible particle release was investigated and discussed. The aim of the present work is to describe the advantages brought by this technology on chemical aspects of the inorganic extractables and delamination propensity. The results obtained with the Alba platform (syringes, vials, and cartridges) will be compared with bulk and spray-on siliconized containers tested with two different analytical methodologies. The first will investigate the inorganic extractables concentrations (i.e., silicon dioxide, sodium oxide, boron trioxide, and aluminum oxide) obtained by an aqueous extracting solution. The second methodology will evaluate the delamination propensity.
Materials and Methods
Samples
The glass containers used in this study were provided by Nuova Ompi S.r.l. (Piombino Dese, Italy) as listed hereunder: 1 mL long luer cone bulk syringes; 1 mL long luer cone spray-on siliconized syringes; 1 mL long luer cone Alba syringes; 3 mL bulk and Alba cartridges; 3 mL bulk and Alba vials. The same bulk containers were used as the base for the Alba and the spray-on siliconized categories. The glass raw material was borosilicate type I glass tubes (thermal expansion coefficient of 51) provided by the same vendor; the composition of the glass is reported in Table I.
Chemical Composition of the Glass Tubes
Bulk syringes, packed in an ISO 8 cleanroom after the forming and annealing steps, were rinsed with water for injection (WFI) and dried. The spray-on siliconized syringes were processed, starting from the same bulk product, in ISO 5 conditions; the siliconization was realized with 0.5 mg/barrel Dow Corning 360 medical fluid 1000 cts.
Alba containers were produced in an uncontrolled laboratory environment; the only precaution taken to reduce the possible external contamination was the use of an ASALAIR 1200FLV laminar flow cabinet (ASAL S.r.l., Milan, Italy). Alba technology is an innovative coating process that is described in a pending patent (18). After washing (with WFI) and drying the containers, the inner surfaces are sprayed with a silane coupling agent and the containers are baked to fix the pretreatment. The silicone, which is the same Dow Corning 360 medical fluid 1000 cts used for the standard spray-on siliconized containers, is then sprayed-on and is cross-linked with the aid of an atmospheric plasma.
Materials
Calibration curves for the Inductively Coupled Plasma (ICP) instrument were obtained using 1000 mg/L standard stock solutions of Al, B, Si, Na, Ca, K, and Y (used as an internal standard) provided by Merck (Darmstadt, Germany).
Grade I deionized water was obtained from the Millipore ELIX system (Milli Q IQ7000, Merck Millipore, Burlington, MA) and used as the filling solution. Its purity corresponds to what is prescribed by the European Pharmacopoeia for the water R1. Sodium chloride 99.9% w/w (Sigma-Aldrich, St. Louis, MO) was used to prepare the delamination extracting solution and sodium hydroxide (Sigma-Aldrich) was utilized for pH adjustments.
In method A, the deionized water was used as the filling media for the containers and for the preparation of calibration curves of Al, B, Si, Na, Ca, and K, by addition of the appropriate aliquots from commercially available standard stock solutions (1000 mg/L). Intermediate quality control (QC) solutions were used for quality verifications. Yttrium stock solution (1000 mg/L) was used as internal standard (final concentration 1 mg/L). All solutions were acidified with 0.1% HNO3 (purity for trace metal grade analysis).
In method B, a 0.9% NaCl solution was prepared from 99.9% w/w sodium chloride, except for the filling media, and adjusted to pH 8 with a few drops of diluted NaOH solution. This media was used throughout as extractant in delamination testing. For the ICP analysis, NaCl was used as a zero-member solution and in the preparation of calibration curves for the determination of Al, B, and Si. Intermediate QC solutions were used for quality verifications. All of the solutions were acidified with 0.1% HNO3 (purity for trace metal grade analysis).
Instrumentation
To accelerate the effects of the glass contact with the extracting solutions (methods A and B), a physical and thermal stress was introduced with the use of a TL24 Autoclave (De Lama, Italy). After the autoclave treatment, the extracted solutions were analyzed with an ICP-OES iCAP 7400 (ThermoScientific, Waltham, MA).
Only for test method B was the integrity of the inner glass surfaces also investigated by methylene blue staining and by scanning electron microscopy (SEM), details of which are given below at the end of the description of test method B. The surface characterization was performed using a Zeiss Sigma variable pressure field-emission scanning electron microscope (VP-FE-SEM) with 1.5 nm of maximum resolution.
Test Methods
The same autoclaving cycle (1 h, 121°C) was used as stress treatment for both test methodologies. The two treatments differ in the extracting solution. The use of water as extracting media for test method A was suggested by the indications and guidelines given in the Annex of EP 3.2.1 (19), while the use of a more aggressive solution (NaCl pH 8) for test method B was suggested by USP <1660> Evaluation of the Inner Surface Durability of Glass Containers (20). The details of the procedures are given below.
Test Method A (Water as Extraction Solution):
All the samples were initially rinsed three times with distilled water and allowed to drain. To fill the containers, the cone of the syringes was closed with 8550NR Aptar Stelmi RNS (rigid needle shield), the mouth of the cartridges was crimped with Lined Caps (LC) 7236, 4780/7778 (West, USA), and no rubber was used for the vial format. All the rubber components had been previously decontaminated by applying the procedure used by Guadagnino et al. (21), but measuring and verifying the eventual residual contamination were done using as analytical instrument the ICP-OES instead of flame atomic absorption spectroscopy (FAAS). This change allowed a reduction of the target limits, which depend on the limits of quantification (LoQ) of the instrument, for the Al, B, Na, Ca, and K elements to 0.05 ppm and for Si to 0.1 ppm.
The glass containers were filled with Milli Q water up to 1.3 mL for the 1 mL long syringes, 3.6 mL for the 3 mL vials, and 3.6 mL for the 3 mL cartridges. These values of filling volumes had been determined with the procedure described in ISO 4802-2, part 7.2 Determination of the Filling Volume (22) for vials and cartridges, while for the syringes, the same volume as the one utilized for the particle analysis described in the previous publication was used in order to have comparable data. After filling, all the containers were covered with an aluminum foil previously decontaminated by rinsing with acetone and distilled water. The closed samples were placed in the autoclave in a vertical position with the flanges, back ends, and mouths up for the syringes, cartridges, and vials, respectively. A calibrated thermocouple inserted into a container, which had a volume comparable to the samples subjected to the analysis, filled with distilled water and covered in turn with a decontaminated aluminum foil, was used to monitor the temperature during the autoclave treatment. An autoclave cycle at 121°C for 1 h was performed as described in the Ph. Eur. 3.2.1 (19), after which the samples were removed from the autoclave and were left to cool down at room temperature within 30 min. The extraction solutions were carefully transferred through the flange, the back end, or the mouth in the case of syringes, cartridges, or vials, respectively, into particle-free plastic test tubes. In the case of the syringes, the extracted solution was diluted 1:2 as follows: from each test tube, 1 mL of solution was sampled and transferred into a new test tube, where it was diluted with 1 mL of 0.1% HNO3. After thoroughly stirring the tubes at low speed for 2 s to homogenize the solution, their content was fed into the ICP-OES instrument for analysis.
Test Method B (NaCl as Extraction Solution):
Test method B is similar to test method A, but it is performed with a high ionic strength solution, which is known to cause more glass corrosion (20). After washing the containers as described earlier, they were filled with a solution of 0.9% w/v NaCl adjusted to pH 8.0 with a diluted NaOH solution. The same autoclave cycle of test method A was then performed on the containers. Before the ICP analysis, the syringe-extracted solutions were diluted by adding 1 mL of 0.9% NaCl pH 8.0 solution to 1 mL of extracted solution.
After the autoclave treatment, in addition to the ICP analysis, the level of damage and corrosion of the inner surface was investigated. For this reason, the inner surface of the containers was subjected to SEM scanning and a methylene blue staining test.
SEM analysis was performed after fracturing the samples by mechanical pressure to expose the inner surface. The samples to be scanned were fixed on aluminum stubs with a carbon double tape and inserted into the sample chamber. The following parameters were utilized to minimize artifacts and maximize the resolution: working distance between 2 and 4 mm, beam energy of 1 keV, and the aperture with a diameter of 30.00 μm. For this study the instrument was used in high vacuum mode with no metal coating needed on the samples.
Methylene blue is a cationic dye used commonly to test glass corrosion. It is thought that the positive ions of the dye dissociate in the aqueous solution and bond with the glass through ion exchange (23), highlighting where the glass network was damaged. This destructive staining test, performed in addition to the SEM analysis, was carried out as follows: The containers were rinsed three times with deionized water, filled with a 0.5% w/v methylene blue aqueous solution and emptied after 20 minutes. To eliminate the excess dye, the samples were rinsed three times with deionized water and dried for 20 min in an oven (STF-F, Falc Instruments S.r.l, Treviglio (BG), Italy) set to 105°C. The containers were then inspected with the naked eye; if the glass retained a noticeable blue coloration, it indicated that that part of the glass presented some corrosion.
Results and Discussion
Samples have been characterized with two different test methods in order to determine both the extractables profile (test method A) and the delamination propensity (test method B).
Extractables Profile
Test method A was performed on three different groups of containers (1 mL long syringes, 3 mL vials, and 3 mL cartridges) with 50 samples analyzed per category. The results, expressed as oxide equivalents, are detailed for Al2O3, B2O3, SiO2, and ΣNa2O in Table II; the “ΣNa2O” column indicates the amount of sodium oxide summed to the potassium and calcium oxides converted, with the appropriate coefficients, into sodium oxide equivalent, as indicated in ISO 4802-2, part 9.1 (22). The LoQ for the various elements and for the dimension of the containers (the extracting solutions of the 1 mL syringes had to be diluted before the analysis) are illustrated in Table II. The concentrations of inorganic components extracted from Alba containers are significantly lower than those for the bulk products and, for the specific case of 1 mL long syringes, also lower than those for the spray-on siliconized category. The results reported in Table II highlight a significant low standard deviation on Alba-coated syringes. The spray-on siliconized syringes showed comparable ΣNa2O extraction but higher SiO2 concentration than the bulk syringes, because the silicone particles detached from the barrel ended up in the extracting solution, contributing to the final SiO2 value.
Results of Test Method A Applied on Syringes, Vials, and Cartridges Expressed in parts per milliona
All of the analyzed Alba formats present a strong reduction of inorganic extractables compared to the corresponding bulk containers, confirming the barrier effect property of such innovative technology; for the SiO2 and ΣNa2O, the reduction is >70%. The comparison cannot be performed for the other oxides (Al2O3 and B2O3), as for Alba, the concentration is below the LoQ.
Stability of Alba Coating
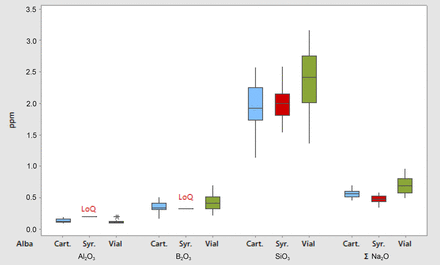
The coated containers present similar extractables results, where the data are presented as boxplots to compare the distributions of the three categories, as shown in Figure 1. The values of Alba vials were slightly higher than the values obtained from Alba cartridges and syringes; these results derive from the different surface area to volume ratios and the different conversion processes to form the vials compared to the syringes. In particular, the bottom forming process has a higher impact on the total amount of extractables.
Inorganic extractables boxplot for the Alba containers (water extraction).
LoQ = Limit of Quantification.
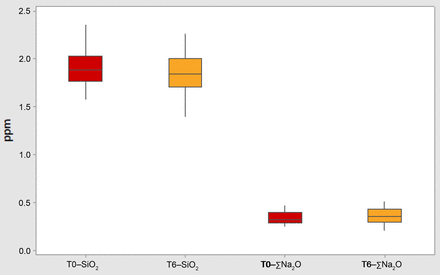
An accelerated aging study was performed to test the stability of the Alba coating on syringes. As discussed in the previous publication about the Alba platform (17), the 1 mL long syringe was chosen as the container typology to use for a complete stability study; as a matter of fact, it is one of the most challenging containers for both the analytical methodology and the coating performance owing to its small filling volume and its high surface area-to-volume ratio. The ICH guidelines (24), SADC guidelines for stability testing (25), and the ASTM F1980-07(2011) standard (26) were used as reference conditions. The values of inorganic extractables obtained from testing 30 syringes immediately after production (T0) are compared to those obtained from 30 Alba syringes that were stored empty and in a vertical position with the flanges up for 6 months in a certified climatic chamber at (40°C ± 2°C) and (75% ± 5%) relative humidity (RH). The results for the SiO2 and ΣNa2O – the other elements are not reported, as they were below the LoQ values – are reported in Figure 2 as boxplots to compare not only the average values but also the two distributions.
Boxplot of concentrations of silica and sodium oxide for Alba syringes measured immediately after the autoclave cycle and after 6 months of storage and autoclave.
Looking at the data, the 6 months of accelerated aging seem to not have an impact on the amount of released SiO2 and ΣNa2O. To confirm this observation, a statistical approach was applied on the T0 vs T6 ppm SiO2 results and on the T0 vs T6 ppm ΣNa2O results, verifying the distributions normality and consequently applying the appropriate tools to verify the comparability of the means and the variances of the populations. Because all the data resulted from a normal distribution, the 2-samples t-test was used to compare the means, while Levene's test was used to verify the variance comparability. The results of this statistical evaluation (not shown here) proved that the means and the variances of the SiO2 and ΣNa2O released from the containers before and after 6 months of storage are comparable, thus confirming the stability of the coating under accelerated conditions.
Delamination Propensity
The same three categories of containers were also analyzed following the delamination propensity procedure. The scope of this analysis was to compare the delamination propensity of the Alba platform containers with the corresponding bulk ones. Moreover, the sprayed-on siliconized syringes were tested in order to have a reference value for the siliconized containers. In comparison with the extraction in aqueous solution performed before, the test with NaCl solution at pH 8 was more aggressive for the glass surface. In fact, this method can be used to predict and rank an early propensity of the glass vials to delaminate, long before the appearance of flakes in the solution as was shown in a previous publication (9). Samples are characterized after the autoclave cycle, both quantitatively with atomic emission spectrometry and qualitatively with a methylene blue staining test; the outcomes pointing toward a delamination propensity were further examined with SEM.
As expressed before, the comparison has been conducted on three categories: vials, cartridges, and syringes. Vials are quicker to delaminate owing to the bottom forming process, with volatilization of the components and their deposition on the inner surface.
The results from the ICP analysis are shown in Table III; the column for ΣNa2O is not present because of the type of extractive media (NaCl solution).
Results of Test Method B Applied on Syringes, Cartridges, and Vials Expressed in parts per milliona
It can be observed that in general the concentration values are higher than the ones obtained with water as the extraction solution shown in Table II. This was expected, as the NaCl solution is a more aggressive media and therefore the magnitude of the inorganic elements dissolved in the extracting solution increases.
As was observed for test method A, the percentage differences between the Alba containers and the corresponding bulk formats is >70% for SiO2. For the other measured oxides, the reduction is approximately between 60% and 80% based on the specific case. The reduction of the oxides concentrations demonstrates, also in this case, the barrier effect property of the Alba coating independent from the type of format.
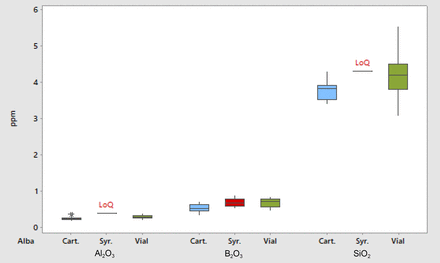
ICP analysis, conducted as previously described, allows analysis of the different containers in the Alba platform in order to investigate the differences in the amount of extractables among Alba syringes, cartridges, and vials. The results are shown in the boxplot presented in Figure 3; extractable values are similar among the various containers. In addition to the ICP analysis, a methylene blue staining test was performed on five syringes and vials before and after the autoclave stress treatment. The glass acquired a blue hue where higher surface porosity was present on the container and, conversely, remained transparent where the glass structure was intact. Representative pictures of the test are presented in Table IV: one representative container for each category is shown. For each sample, two pictures are displayed: the pictures on the left are the originals, while those on the right have been similarly post-processed to highlight and to better appreciate the blue staining. The post-processing method applied on the picture was the same for each sample to make the “enhanced” images comparable between each other's images.
Boxplot of inorganic extractables for three different containers in the Alba platform (NaCl extraction).
LoQ = Limit of Quantification.
Outcomes of The Methylene Blue Test Before and After Being Subjected to the Autoclave Stress of Test Method Ba
It is worth noting that the syringes did not present any significant spot highlighted by the methylene blue test either before or after the autoclave treatment. In this case, no visible differences are noticeable between bulk and Alba syringe containers, and the test can be considered negative—no substantial staining—for all of the samples. This is not surprising as the syringe forming process does not negatively impact on the glass durability (27).
The bulk vials result clearly indicates that in this case there is a specific area, the heel of the vial, which has been most affected by the extraction solution (Table IV). As a matter of fact, when the bottom of the glass vial is formed from the glass tube, the involved high temperatures cause a migration to the surface, followed by an evaporation and consequent re-condensation, of the highly volatile alkalis and borates. This causes a change in the chemical composition of the bottom area, making it particularly sensitive to alkaline attacks as in this case with the pH 8.0 NaCl solution, which can eventually induce delamination. Because heel staining was expected, and present in the bulk case, it is interesting that the Alba-coated vials do not show traces of methylene blue. It is possible to imagine that the Alba coating formed a protective barrier on the glass of the vials, substantially reducing the corrosive attack of the extracting solution.
Methylene blue staining is a quick and easy test; its outcomes, considered in the light of the ICP results, can provide an indication of the level of the glass corrosion in addition to the information of where the corrosion took place. This information, nonetheless, is not a conclusive evidence that there is a delamination propensity; it has to be confirmed with a direct investigation of the internal surfaces, like with the SEM micrographs as was done in this study.
Because the syringes were not stained by the methylene blue, they were not analyzed by SEM, while for the vials, an in-depth SEM analysis was performed. Table V shows some illustrative images of the bottom part of the bulk and Alba vials, both before and after NaCl autoclave treatment. They clearly indicate that the bottom part of the bulk vial barrels was deeply affected by the extracting solution. In particular, the bulk surface was characterized by lens-shaped droplets before the treatment (Table V), while after the autoclave treatment, numerous depressions appeared, signaling that the solution corroded the surface of the glass. Corrosion can possibly lead in time to delamination if the glass is extensively in contact with an aqueous solution (14), but no cracking on the surface was observed, indicating that the vials did not start the process of delamination after the contact with the pH 8 NaCl solution. The images show that the craters are absent on Alba samples and that the silicone oil-based coating covered the glass both before and after the autoclave treatment. This confirms the methylene blue results. The Alba coating provides a shield between the glass and the extracting solution.
SEM Micrographs Taken at the Bottom of Alba and Bulk Vials Before and After Autoclaving Cycle
Conclusions
In this study, the release of inorganic extractables and the delamination propensity of the containers belonging to the Alba platform were examined using dedicated testing protocols.
The results of inorganic extractables showed a decrease of 50–70% (based on the oxides type) for Alba containers in comparison with their respective bulk containers. All the containers included in the Alba platform showed comparable results. Interestingly, the SiO2 content remains considerably lower in the Alba containers, although the coating is silicone oil-based.
The results of delamination propensity testing showed that the Alba coating creates a barrier effect that reduces the extraction values of SiO2, Al2O3, and B2O3, creating a surface not impacted by contact with an aggressive alkaline solution and thus considerably reducing the degree of corrosion and the risk of delamination.
All containers belonging to the Alba platform, i.e., syringes, cartridges, and vials, showed superior performances. Even though during the development phases the container needed modification, the drug would always face the same surface. This could greatly reduce the drug formulation development time, facilitating the transition from one container to another and considerably reducing the risk of drug product incompatibilities; this impacts stability by requiring a reduced number of tests, and also regulatory aspects with a higher probability of acceptance by health authorities.
Conflict of Interest Declaration
The authors herewith declare that they do not have any financial or non-financial competing interests related to the content of the manuscript.
- © PDA, Inc. 2019