Abstract
Endotoxins, heat-stable lipopolysaccharides from Gram-negative bacteria, are potential contaminants that can be introduced during manufacturing of pharmaceutical products, including vaccines. Parental pharmaceutical products undergo endotoxin testing because endotoxins are pyrogenic in humans and can induce severe physiological reactions. Currently, animal-derived Limulus amoebocyte lysate (LAL) assays are widely used. Assays using recombinant factor C (rFC), a nonanimal-derived reagent, have been proposed as alternatives. Some components in the matrices of pharmaceutical products can interfere with these assays. We compared two LAL- and two rFC-based assays for endotoxin detection in four complex human vaccine matrices. We showed that the results for the rFC-based assays were at least equivalent to those for the LAL-based assays, although the rFC-based assays were found to be adequate but slightly less suitable for one of the products that contained proteases as the methods used to inactivate the proteases reduced the assay performance. Likewise, LAL was adequate but less suitable for another product that contained glucans. The rFC assays offer a number of benefits, including compliance with the principles of the 3Rs, i.e., replacement, reduction, and refinement of animal testing by safeguarding animal welfare and promoting more ethical and sustainable use of animals for testing. After they are fully validated, as per the compendial requirements, they could be considered as suitable replacement assays for the detection of endotoxin in the manufacturing processes of pharmaceutical products. In summary, we demonstrated that both LAL and rFC assays are adequate for testing and releasing four vaccine products.
Introduction
Endotoxins are heat-stable lipopolysaccharides (LPSs) from Gram-negative bacteria. When injected into the human body (e.g., via the intravenous, intrathecal, intramuscular, or subcutaneous routes), endotoxin is seen as an indicator of the presence of bacteria by the immune cells, via an innate reaction. These cells respond with a pyrogenic reaction that can be responsible for severe physiological reactions. In addition to inflammation, endotoxins can also induce a septic shock response (1). However, although LPS is the most potent inducer of cytokine production in septic shock, this is not entirely specific to Gram-negative bacteria as septic shock can also be caused by trauma, antigenic immune response, and so forth (2⇓–4). Gram-negative bacteria have been reported to be responsible for 50% of sepsis cases (5). Hence, parenteral products are tested for endotoxin contamination as detailed in various pharmacopeias and specific vaccine monographies (6⇓⇓–9).
For nearly 40 years, animal-derived Limulus amoebocyte lysate (LAL) assays have been widely used for the detection of bacterial endotoxins in the pharmaceutical industry. These assays are routinely used within Sanofi Pasteur for vaccine release testing. In the presence of endotoxin, factor C in the LAL is activated by binding to the endotoxin, and this induces a cascade of reactions (Figure 1) (10). In the kinetic chromogenic LAL assay, a clotting enzyme, via factor G, cleaves a peptide that has the chromogen para nitroanilide (pNA) attached at its terminal to release the pNA, which produces a yellow solution that absorbs light at 405 nm. The time (in seconds) needed to reach a predetermined absorbance of the reaction mixture is measured to determine the concentration of endotoxin. One of the potential drawbacks of the LAL assay is that other substances, such as (1→3)-ß-glucans, which can come from cellulose-based devices commonly used in vaccine manufacturing processes, for example for purification or sterile filtration, can also activate the LAL cascade and thus lead to false-positive results (11). Glucan-blocking buffers can be used to block the factor G pathway of the endotoxin cascade and have been reported to effectively prevent these false-negative reactions (12–13).
Principle of Limulus amoebocyte lysate (LAL) and recombinant factor C (rFC) assays showing that FC is activated by endotoxin in both assays (10).
Recombinant factor C (rFC) is a nonanimal-derived reagent that has been proposed as an alternative to LAL. Recombinant FC assays can be based on fluorescent endpoint assays, with the main receptor being rFC instead of factor C from horseshoe crabs (Figure 1). Unlike in the past, endotoxin rFC assays are now available from different manufacturers, reducing concerns about a single-source supply. In addition, the enzymatic reaction for rFC is much simpler than that for the LAL-based assay, as can be seen in Figure 1. Another potential advantage is the more consistent interbatch performances of rFC assays, which have uniform and predictable behavior toward potential interfering substances with none of the biologic variability inherent in LAL assays, resulting from the production process for rFC. Recombinant FC assays have been shown to be insensitive to (1→3)-ß-glucans, which therefore reduces the rate of false-positive results, and the shorter enzymatic reaction results in less interference from the matrix (14).
The increased demand for endotoxin testing (driven by maturing Asia Pacific markets and growing needs from pharmerging countries) could have an impact on horseshoe crab conservation (15⇓–17). Reducing the use of LAL-based assays would be aligned with the principles of the 3Rs, that is, replacement, reduction, and refinement of animal testing (18). The United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and other regulatory authorities recognize rFC assays as an alternative endotoxin detection method to the prescribed LAL-based assays (7, 19, 20). Since July 1, 2016, rFC assays have been accepted for use as an alternative to the compendial LAL assay as indicated in the Ph. Eur. (20). However, it is necessary to demonstrate that “the method is appropriate for the given substance or product and gives a result consistent with that obtained with the prescribed method …” (21). The alternative assay should be validated as described in USP <1225> Validation of Compendial Procedures in the USA and Chapter 5.1.6.: Alternative Methods for Control of Microbiological Quality in the Ph. Eur. (19, 22⇓–24).
For these reasons, we evaluated four different assays (two LAL-based and two rFC-based) for the detection of endotoxins in four complex human vaccine matrices and we report our findings here.
Materials and Methods
Endotoxin Assays
We evaluated two kinetic chromogenic LAL-based endotoxin assays, Kinetic-QCL (KQCL; Lonza, Walkersville, MD) and ENDOSAFE-MCS (Charles River, Charleston, SC). ENDOSAFE-MCS is a miniaturized, automated version, similar to the KQCL assay, but which uses a multiple-cartridge system and an internally archived standard curve to determine concentrations. Both assays were performed as described in the supplier's instructions. The sensitivities of the KQCL and ENDOSAFE-MCS assays are 50–0.005 EU/mL and 0.5–0.005 EU/mL, respectively.
For the KQCL assay, the released chromogenic product (yellow) was measured at 405 nm with a BioTek ELX808 (BioTek Instruments, Inc., Winooski, VT) absorbance microplate reader coupled with WinKQCL software (Lonza). The samples were tested in duplicate and the mean was used for calculations. The concentrations were determined using standard curves fitted with a polynomial regression (power curve) model.
We also evaluated two rFC assays, ENDOZYME II and ENDOLISA (bioMérieux). The ENDOZYME II assay is a fluorescent endpoint 96-well microplate assay, which was not commercially available at the time of this study, although it is now available. Endotoxin binding activates the rFC to create a moiety that cleaves a synthetic substrate resulting in the generation of a fluorescent compound. The fluorescence was measured with a BioTek FLX800 microplate reader (BioTek Instruments, Inc.) at time zero and after 1 h incubation at 37°C ± 1°C using excitation and emission wavelengths of 380 nm and 440 nm, respectively. The difference between these two fluorescence readings at time zero and 1 h after incubation is proportional to the log10 endotoxin concentration and is linear in the 0.005–50 EU/mL range. Each sample was tested in triplicate and the mean was used for calculations. The concentration of endotoxin in a sample was calculated relative to a standard curve. The standard curve was analyzed using a 4-parameter logistic regression model for 1/10 dilutions from 50 to 0.005 EU/mL (25).
The ENDOLISA assay is a solid-phase, endpoint fluorescence microplate assay using a recombinant bacteriophage-derived capture molecule that has high affinity and high specificity for the conserved core region of LPS that enables it to bind endotoxin variants (26). After the endotoxin was bound on the solid phase, the sample matrix, with potentially interfering components, was removed by washing, thus facilitating reliable quantification of the endotoxin in the sample. The bound endotoxin was then detected using a fluorogenic substrate. The fluorescence was measured with an FLX800 microplate reader at time zero and after 1 h incubation at 37°C using excitation and emission wavelengths of 380 nm and 440 nm, respectively. Each sample was tested in triplicate and the mean was used for calculations. The standard curve was analyzed using a 4-parameter logistic regression model for 1/10 dilutions from 50 to 0.05 EU/mL (26).
Reference Standard Endotoxin
The reference standard endotoxin (RSE) was diluted with 5 mL of endotoxin-free water (vortexed for 30 min as per supplier instructions) to give a final concentration of 2000 EU/mL (batch 5.1: European Directorate for the Quality of Medicines and HealthCare (EDQM)/Council of Europe, France). RSE was used to spike undiluted test samples (hard spike). For this, 50 µL of a 2000 EU/mL RSE solution was added to 950 µL of sample, resulting in 1 mL of spiked sample with an endotoxin content of 100 EU/mL. The control standard endotoxin (CSE) supplied in each assay kit was used to prepare the positive product controls (PPCs) (diluted samples).
Complex Vaccine Matrix Samples
Four proprietary human viral and bacterial vaccines were selected for their different complex matrices (Sanofi Pasteur, Marcy l'Etoile and Val de Reuil, France). The routine release tests for all four had been performed with the KQCL assay. Product A was an attenuated live viral vaccine containing proteases. Product B was an inactivated viral vaccine with no interference for the endotoxin assay. Product C was an inactivated bacterial vaccine potentially containing natural endotoxin content from the Gram-negative antigens it contained. Product D was an inactivated viral vaccine with a strong red color that can interfere with the yellow endpoint product in classical LAL chromogenic kinetic assays.
Assay Conditions
The conditions for each assay were tested before the comparisons were performed. One of these conditions is the dilution necessary to minimize interference (inhibition or enhancement) from matrix substances while respecting the maximum valid dilution (MVD). The MVD is calculated by dividing the endotoxin limit (i.e., the maximum acceptable endotoxin concentration in the undiluted sample) by the assay sensitivity (i.e., the lowest standard concentration, e.g., 0.005 EU/mL for ENDOZYME II). The dilutions used for the KQCL assay were those used routinely in our laboratory. For the other assays, the dilutions investigated ranged from no dilution to 1/10,000 (Table I).
Dilutions Used for Each Product in the Limulus Amoebocyte Lysate- and Recombinant Factor C-Based Assays and Their Sensitivities
We investigated the following pretreatments for the inactivation of proteases: denaturing by heating at 75°C for 15 min, a protease inhibitor, higher dilutions in endotoxin-free water, and dilutions in 0.5 M endotoxin-free NaCl. Product C contained β-1,3-glucan, which is a glucose polymer of varying molecular weight that can be present in preparations derived from yeast and cellulosic material, including hemodialysis filters. When present in sufficient quantities, β-1,3-glucan can produce a positive LAL result in the absence of endotoxins by activation of the glucan-sensitive G pathway (Figure 1). The presence of β-1,3-glucan in Product C was confirmed using the Glucatell assay kit (Associates of Cape Cod, Inc., East Falmouth, MA). Therefore, we added a β-1,3-glucan blocker diluent to block the G pathway and minimize the false-positive reaction.
The dilutions for spiked and unspiked samples were prepared from the same aliquot at the same time for use in all four assays. As a prerequisite for method comparability, three independent runs per method were performed by two technologists with each sample in duplicate for the LAL assays and in triplicate for the rFC assays. Only one run was performed for the investigation of pretreatments for Product A.
The assay validity criteria were the coefficient of correlation r > 0.980 and a percentage of recovery of PPC 50%–200%, based on the Ph. Eur. 2.6.14, USP <85>, and JP 4.01 (6, 8, 9). In addition, the coefficient of variation (CV) for the samples and spikes was <10.00% for KQCL and <25.00% for ENDOSAFE-MCS and ≤25.00% and ≤20.00% for samples with ENDOLISA and ENDOZYME II, respectively, based on the manufacturers' recommendations (8, 9, 25⇓⇓–28).
Outcomes for Comparison of Endotoxin Assays
Using the optimal conditions identified, the assay sensitivities for all four assays were compared. The endotoxin content was expressed in EU/mL. We evaluated the RSE recovery in all four products and in water to calculate the percentage RSE recovery as: 100 × [sample endotoxin reportable results at time t] divided by [water control reportable results at time t]. The average percentage RSE recovery was calculated using the average results in the calculation. The high concentration spike and the PPC recoveries were estimated as the percentage of the added endotoxin that was recovered and had to be between 50% and 200% to validate the test results.
Results
Optimization of Assay Conditions for Product A
Because Product A was known to contain proteases, preliminary assays were performed to assess their impact on the results from the LAL and rFC-based endotoxin assays. We observed a positive signal with only the rFC-based assays: the endotoxin signals for untreated, unspiked Product A were 31 EU/mL and 0.6 EU/mL with ENDOZYME II and ENDOLISA, respectively (Table II). The shapes of the sample rFC curves were different from the shapes of the standard curves, unlike the KQCL curves, which were the same shape. In addition, a signal, corresponding to 0.80 EU/mL, was detected with the ENDOLISA assay in wells containing samples of Product A, with and without the RSE spike, when rFC was not added. This signal was because of the protease in Product A that cleaved the fluorogenic substrate, leading to a false-positive result. The comparison of wells with and without added rFC showed that the potential residual endotoxin was <0.01 EU/mL. This indicates that the false-positive results in rFC were because of nonspecific activation.
Results From One Assay for Samples of Product A (No Pretreatment or Heated to 75°C for 15 min) without and with an Endotoxin Spike
Different pretreatment methods for Product A were therefore tested to minimize the expected impact of the proteases on false-positive results. Heat treatment(75°C for 15 min) to inactivate the proteases did not prevent signals in rFC-based assays and resulted in substantial endotoxin masking in spiked samples, possibly owing to exposure of hydrophobic surfaces by protein denaturation, which would drastically increase particle motility (Table II). Pretreatment of Product A with 0.5 M NaCl did not prevent protease binding to the ENDOLISA microplate. The PPC recovery was 161%, close to that obtained with samples diluted in water (155%), and the decreased endotoxin signal was insignificant (1/10: 0.06 vs. 0.08 EU/mL in 0.5 M NaCl and water, respectively). Six concentrations of benzamide hydrochloride (a serine protease inhibitor) (5, 10, 25, 50, 75, and 100 mM) were tested in the ENDOLISA assay. The maximum concentration of benzamidine hydrochloride that can be tolerated by the ENDOLISA assay is 100 mM and concentrations of 1–10 mM are sufficient to inhibit most serine proteases. There were no significant differences between the results when Product A was diluted in water or in benzamidine hydrochloride, suggesting that either the benzamidine hydrochloride was washed off during the assay or that the protease present was not a serine protease.
When Product A was diluted 1/100 and 1/10,000 in water for the ENDOLISA and ENDOZYME II assays, respectively, the assay results were <5 EU/mL and <50 EU/mL, respectively. When Product A was diluted to 1/100 in 0.5 M NaCl for the ENDOZYME II assay, the PPC recovery was <50%. The results for spike recovery (PPC) were within the prespecified limits for all four methods in the samples without and with heat treatment (Table II).
None of the other pretreatments evaluated for Product A (i.e., higher dilutions in LAL reagent water, dilutions in 0.5 M endotoxin-free NaCl, use of antiprotease) removed the signals. However, the ENDOLISA assay gave lower results than the ENDOZYME II assay, probably because the washing steps removed the protease (about 50 times more efficient).
Sensitivity of Endotoxin Detection Assays
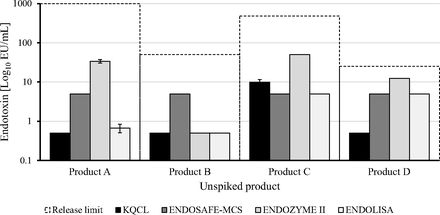
The results obtained with all four endotoxin detection assays were below the required internal product specifications for all four products, with the most sensitive assays being KQCL in Product D and water, and ENDOZYME II in Products A and B and water (Figure 2). ENDOSAFE-MCS gave the least sensitive results in Products A and B, and ENDOZYME II in Products C and D.
Sensitivity of all four endotoxin assays in complex human vaccine matrices and water. The product-specific release limits were 1000, 50, 480, and 25 EU/mL for Products A, B, C, and D, respectively.
Percentage Recovery of Reference Standard Endotoxin
The recovery of PPC from water was 70%, 178%, 69%, and 106% with the KQCL, ENDOSAFE-MCS, ENDOZYME II, and ENDOLISA assays, respectively. The percentage recovery of RSE from water (vs. the theoretical recovery) was 92%, 67%, 84%, and 75% for the KQCL, ENDOSAFE-MCS, ENDOZYME II, and ENDOLISA assays, respectively. The percentages of RSE recovered from the vaccines, calculated as a percentage of the recovery from water, were all within the required limits of 50% to 200% (Figure 3). The average RSE recovery for Product B was >100% for all four assays and was >180% with the KQCL and ENDOZYME II assays.
Percentage recovery of reference standard endotoxin from the four products, calculated as the percentage recovery from water, with the four endotoxin detection assays.
Endotoxin Content Detection
With the exception of Product C with the KQCL assay, all endotoxin concentrations were below the assay's quantification limit (Table III). For Product C, an average endotoxin concentration of 10 EU/mL was obtained with the KQCL assay, but the concentrations were below the sensitivity threshold with the other assays, suggesting a potential enhancement of endotoxin concentration by (1→3)-ß-glucans or another component (one of the vaccine valences is produced on yeast). In the presence of a ß-glucan blocker, the endotoxin concentration detected was, on average, 41% lower in unspiked Product C with three independent runs of the KQCL assay. The mean PPC was 59%, which is within the variability of the method (i.e., 50%–200%). Using the Glucatell assay, we found 153 pg/mL of beta-glucans in Product C. An average endotoxin concentration of <5 EU/mL was obtained with the ENDOSAFE-MCS assay. The differences in result obtained with the LAL-based assays could be because of different lysate sensitivity to reactive material such as (1→3)-ß-glucans (13). The average PPC for Product D was >90% in all assays except for the KQCL assay where it was 57%, which, although low, is a valid result.
Endotoxin Concentrations and Mean Percentages of Spike (Positive Product Control; PPC) Recovery (As a Percentage of Recovery from Water) for Each of the Four Assays with the Four Products (Product A, Unheated). The Results Shown Are the Average Results from Three Independent Runs by Two Technologists
The endotoxin concentrations detected by the assays in all four unspiked products were all below the company's internal product-specific release limits, although the endotoxin concentrations were >10 EU/mL with the ENDOZYME II assay for Products A, C, and D (Figure 4). The highest endotoxin concentration with the KQCL assay was in Product C (10 EU/mL) compared with <1 EU/mL for the other products, but this was below the company's internal product-specific release limit.
Endotoxin quantification in the four unspiked products with the four endotoxin detection assays.
Discussion
Our results showed that the rFC-based endotoxin assays, ENDOZYME II and ENDOLISA, are suitable for the detection of endotoxin in four products with different matrix characteristics. There was no trend for either the LAL or rFC assay results to be higher or lower in the unspiked products, although false-positive results are possible for both as discussed following. The RSE spike recovery (100 EU/mL) was within the required limits of 50% to 200% for all products and all assays. The RSE spike recoveries in the ENDOSAFE-MCS and ENDOLISA assays were closer to 100% than those for the other two assays and, compared with the ENDOLISA assay, the recovery with the ENDOSAFE-MCS assay was higher, possibly owing to the use of an internally archived standard curve in the cartridge.
The results obtained with the LAL-based and the rFC-based assays were within the company's internal product specifications. One advantage of the rFC-based assays is that the presence of β-1,3-glucan, which can come from the manufacturing processes for these products, was not detected, unlike in the LAL tests in which a glucan blocker needs to be added to prevent false-positive signals. We showed that all four endotoxin platforms could be used for the different products tested (Table IV). They all provided results that were below the company's product-specific release limits. In addition, they achieved the Pharmacopeia and Sanofi Pasteur internal assay validity criteria, although some assays were more suitable for some matrices owing to the absence of false-positive results and lower sensitivity to interference from the matrix.
Summary of Recommended Assay Taking into Account the Characteristics of the Different Human Vaccine Matrices
False-positive results can be obtained with LAL assays owing to various factors such as the presence of blood products, polynucleotides, and (1→3)-ß-glucans (13, 29, 30). (1→3)-ß-glucans contain glucose polymers of varying molecular weight linked primarily through (1→3)-ß glycosidic linkages. If sufficient amounts of glucans with a particular molecular weight are present, a positive LAL response, that is not an endotoxin-mediated response, may be observed. The factor G pathway is activated most efficiently by linear (1→3)-ß-D-glucans whereas chains containing branches are less effective, and short oligosaccharide chains (two to seven glucose units) do not activate factor G at all (4, 31, 32). A ß-glucan blocking effect was first reported for a high-molecular-weight ß-glucan, and then a low-molecular-weight ß-glucan was also reported as a ß-glucan blocker (33). The factor G pathway can be blocked by the addition of low-molecular-weight glucans, which can competitively block the LAL alternate pathway at specific concentrations (US Patent 5,155,032) (4, 34).
We showed that the signal observed for Product A in the rFC-based assays (ENDOLISA and ENDOZYME II) is because of the presence of proteases. This is supported by the “positive” result obtained in the absence of rFC. It is possible that assessing different pretreatments for Product A with the ENDOLISA and ENDOZYME II assays could improve the results. For example, the impact of protease on the results with ENDOLISA could be reduced by higher dilutions of Product A in water, which would result in a weaker signal, but this would not completely eliminate the interference from the presence of proteases. Although the result for the unspiked or spiked sample could have been subtracted from the sample tested without added rFC to improve the assay sensitivity in this setting, it would not be feasible to do this routinely for product release in a quality-control laboratory. Thus, the ENDOZYME II was not suitable for testing Product A under the conditions tested. Because the LAL-based assays worked well for Product A, it would seem that the rFC-based assays may not be suitable for this particular Product A protease-containing sample without further testing.
The RSE recovery from Product B was >180% with KQCL and ENDOZYME II, indicating important interference from the product matrix in these two assays and making them less suitable for this product. All assays can be used for Product C. Product D showed an inhibitory effect in the KQCL assay, although the spike recovery was valid (>50%). This is because of the matrix formulation, which contains a red substance that interferes with the measurement of the yellow chromogenic product, making the KQCL assay less suitable.
The RSE recovery from water was best with the ENDOLISA assay, 106% compared with 69%, 70%, and 178% with the ENDOZYME II, KQCL, and ENDOSAFE-MCS assays, respectively. This could be because of the different concentration range that is used for the ENDOLISA assay, which requires a higher PPC spike of 5 EU/mL vs. 0.5 EU/mL with the other assays, as the higher spike can give a better percentage recovery. For the ENDOSAFE-MCS method, the 0.78 EU/mL spike is not added by the operator as it is already present in the cartridge. The high percentage of overlap in water could be because of the cartridge batch used or the technique itself.
We also demonstrated that there was less interference from the matrices in the ENDOZYME II assay than in the KQCL assay. However, it is difficult to compare the levels of interferences from the matrices with the different methods because we also observed differences with the water controls owing to the parameters of each assay, such as the value of PPC and the preparation of standard curve, which were not identical.
Results from one multicenter study showed that the requirements for validation of compendial methods for specificity, precision, accuracy, linearity, range, and quantitation limit were satisfied for the rFC assay and were equivalent to the USP photometric procedures for endotoxin measurement (35). In another study, an rFC-based endpoint fluorescence assay was validated and compared with a LAL-based assay using 10 drug products, 6 drug substances, 2 pharmaceutical formulation excipients, and pharmaceutical production grade water (14).
A summary of the characteristics of the four assays compared in this study, based on our results and information from the manufacturers, can be found in Table V. One of the main advantages of the rFC-based assays is their enhanced specificity for endotoxin. The spike recovery is statistically more robust and their sensitivity range, 0.005 EU/mL to 5 EU/mL, is suitable for endotoxin detection for pharmaceutical products (29). They also do not require samples to be substantially diluted and will, therefore, potentially reduce the number of invalid results arising from the high dilutions required for the KQCL assay. The ENDOSAFE-MCS assay can provide rapid results (e.g., 20 to 30 min for 5 samples with one module) if only a few samples need to be tested, however microplates have higher throughput and up to 21 samples can be tested using one microplate. The ENDOLISA assay takes the longest time to perform, but it is suitable for samples with matrices that have high interference and it is, therefore, the most adapted for troubleshooting purposes.
Comparison of Characteristics for Two Limulus Amoebocyte Lysate (LAL; KQCL and ENDOSAFE-MCS) and Two Recombinant Factor C (ENDOZYME II and ENDOLISA) Endotoxin Assays, Using Information from the Assay Manufacturers and from This Study
The growing need for endotoxin assays has led to fears of excessive demands for LAL and thus potential pressure on the horseshoe crab populations from which the LAL is obtained. When rFC-based assays became available, there was only one commercial supplier of rFC reagents, but now there are other suppliers. Hence, rFC being a nonanimal-derived reagent can help to overcome any potential shortage of LAL while supporting the aims of the 3Rs initiative in terms of more ethical and sustainable use of animals for testing (36).
The pharmacopeia requires that replacement assays are more scientifically relevant, which is the case for the rFC assays as they show high specificity and have a simpler enzymatic reaction than the cascade reaction in LAL-based assays and should, therefore, be less sensitive to interference (37). In January 2019, the Ph. Eur. launched a public consultation on a new General Chapter, 2.6.32: Test for Bacterial Endotoxins Using Recombinant Factor C (rFC). For the time being, this will not be referenced in individual monographs but will be a standalone chapter (38). In addition, the American USP Microbiology Expert Committee organized a workshop in June 2019 to consider new reference endotoxin standards and the requirements for the inclusion of new endotoxins test methods (39).
Conclusion
To conclude, our results from this study demonstrated that the results from the rFC-based assays, ENDOZYME II and ENDOLISA, gave comparable results to those obtained with LAL-based assays. However, samples containing proteases that can liberate the colored substrate or fluorophore in the absence of endotoxin are problematic for both the LAL-based and the rFC-based endotoxin assays. The rFC-based assays offer a number of additional benefits and can be considered as suitable alternative assays for the detection of endotoxin. Our results confirmed that all four methods (two LAL, two rFC) are essentially comparable and are suitable to release all four products.
Conflict of Interest Declaration
All authors are employees of Sanofi Pasteur.
Acknowledgments
This study was funded by Sanofi Pasteur. The authors would like to thank Thomas Uhlig and Sylvie Genoux (bioMérieux, France) for technical assistance. They also acknowledge medical writing and editorial services provided by Margaret Haugh (MediCom Consult, France) and funded by Sanofi Pasteur, and Sandrine Buisson (Sanofi Pasteur, France) for editorial assistance and manuscript coordination.
- © PDA, Inc. 2020