Abstract
Viral inactivation has been demonstrated to be an effective viral clearance step in the biologics purification processes. In the 2019 Viral Clearance Symposium, the topics were focused on alternative eco-friendly and cost-effective detergents, validation of on-column viral inactivation, and further understanding of low pH and solvent/detergent viral inactivation. Sugar-based surfactants and a synthetized replacement of Triton X-100 were evaluated to be effective and robust in inactivating enveloped viruses. For low pH viral inactivation, consistent pH measurement, through alignment of pH meters and probes across different labs and manufacturing facilities, was confirmed to be critical to ensure both product quality and safety. For the well-established solvent/detergent inactivation, an approach of adding premixed solvent/detergent stock significantly improved operation. Different viral clearance approaches were discussed for on-column viral inactivation using a detergent-containing wash buffer.
- Viral inactivation
- Low pH viral inactivation
- Solvent/detergent viral inactivation
- On-Column viral inactivation
- Sugar-based surfactants
- Alternative detergents
- Viral clearance
- Viral Clearance Symposium
Background
Viral inactivation (VI) has been demonstrated to be an effective viral clearance (VC) step within recombinant protein purification processes. Extensive data on VI using both low pH conditions and chemicals were presented in the previous Viral Clearance Symposia (2009, 2011, 2013, 2015, and 2017). In the 2019 Viral Clearance Symposium, six presentations were focused on alternative eco-friendly and cost-effective detergents, validation of on-column VI, and further understanding robustness as well as operation and other factors of low pH and solvent/detergent VI.
Sugar-Based Surfactants for the Inactivation of Enveloped Viruses (Allison Titong, Nicholas Spadoni, Wensheng Wang, and Shengjiang Liu; Bayer)
Sugar-based surfactants (SBSs), N-methylglucamide (Mega) derivatives, were systematically investigated for the inactivation of enveloped viruses spiked in various protein matrices. These green chemicals are biodegradable, have low environmental impact, and have a renewable origin. The specific SBS detergents assessed in this study were octanoyl-N-methylglucamide (Mega 8), nonaoyl-N-methylglucamide (Mega 9), decanoyl-N-methylglucamide (Mega 10), and dodecanoyl-N-methylglucamide (Mega 12), and each was evaluated for virus inactivation at solution concentrations ranging from 0 to 3.7%, at 2°C–25°C for 0–4 hr. The model viruses used for testing were species from Retroviridae (xenotropic murine leukemia virus, X-MuLV), Herpesviridae (porcine pseudorabies virus, PRV), and Flaviviridae (bovine viral diarrhea virus, BVDV). Virus inactivation capacity was determined by the tissue culture infectious dose (TCID50) assay of SBS-treated samples compared with that of untreated controls.
The results demonstrated that SBSs (Mega 8, Mega 9, Mega 10, and Mega 12) were highly effective in inactivating enveloped viruses at 2°C and ambient temperatures within 30 min of incubation at each respective critical micelle concentration (CMC). SBS (Mega 10) virus inactivation is equally effective in the various protein matrices including manufacturing intermediates of recombinant human factor (rhFVIII), human immunoglobulin G (IgG), and plasma protein. Furthermore, based on a chromogenic potency assay, rhFVIII activity was maintained in SBS treatment and comparable to that of untreated controls, indicating that SBS detergents have no impact on protein function.
Taken together, SBS detergents are robust at enveloped virus inactivation and are ideal candidates for application in the purification process for biological manufacturing.
pH Measurement Considerations across Development, Pilot Plant, and Manufacturing Sites (Richard DJ Chen; Eli Lilly and Company)
Low pH inactivation (LpH) is typically incorporated as a robust orthogonal virus reduction unit operation for monoclonal antibody (mAb) purification processes. A discrepancy in titrant volume addition for two separate studies prompted an investigation into the pH meters and probes used within both development and manufacturing.
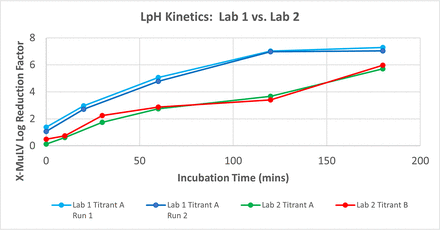
A LpH VC study was executed at a contract testing site (Lab 1) to support early-phase clinical materials for a mAb. Prior to the pivotal campaign, a titrant change from Titrant A to Titrant B was proposed to reduce volume expansion. A bridging study was completed at Lilly (Lab 2) to verify that inactivation kinetics were not impacted by the titrant change. Figure 1 summarizes the results from the two VC studies. Table I summarizes pH and titrant volume data from the two VC studies as well as the good manufacturing practices (GMP) pilot plant run. Titrant ratio is the titrant volume added to reach the target pH over the starting volume before titration, for example, a 0.988 titrant ratio represents the addition of 0.988 mL of titrant to a starting volume of 1 mL.
LpH kinetics: Lab 1 vs Lab 2. LpH, low pH inactivation; X-MuLV, xenotropic murine leukemia virus.
pH and Titrant Volumes
Based on the results from both VC studies, the titrant change did not impact LpH performance. The kinetics and overall log reduction factor (LRF) were identical for the two runs performed at Lab 2 using both Titrants A and B. In addition, less Titrant B was needed to reach the target pH compared with Titrant A, which was expected because volume expansion reduction was the reason behind the titrant change. Lastly, there was a >1 LRF difference between the results from Lab 1 vs Lab 2 suggesting titration differences due to pH measurement differences between Labs 1 and 2.
Low pH parametric data from the Pilot Plant is also provided in Table I as a reference point. At the Pilot Plant scale, more titrant volume was needed for pH adjustment because the target pH in manufacturing before the incubation hold was lower than the target pH of the small-scale VC runs. A higher target pH (3.60) was implemented for the small-scale VC studies to simulate worst-case conditions to cover the full operating range for manufacturing.
To further investigate the load ratio and LRF discrepancies observed, a separate development study was completed to assess measurement differences between pH probes/meters used across development labs and commercial manufacturing at Lilly. Three titrant samples were generated (Table II) and measured using six different pH probes/meters, as shown in Table III.
Titrant Samples for Measurement
pH Probes/Meters Used in Development and Manufacturing
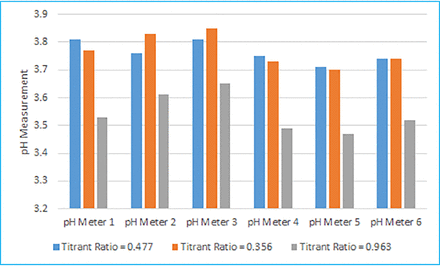
The results of the development study are summarized in Figure 2. For the three titration samples, pH measurements from the various pH probes/meters were within 0.3 pH units of each other. However, it should be noted that the relationship between titrant volume and starting pH is not linear, for example, the titrant volume needed to titrate a sample from pH 3.8 down to 3.7 vs 3.6 could be significantly over double depending on the buffer matrix and titrant. Overall, pH probe/meter #3 provided the highest readings and pH probe/meter #5 providing the lowest readings. Multiple calibration methods (e.g., 2-point calibration, 3-point calibration, and so forth) were assessed and determined to not have a significant impact on pH measurements (<0.1 pH measurement difference). The pH measurements of Samples #2 (titrant ratio [TR] = 0.477) and #3 (TR = 0.356) for each probe/meter in Figure 2 can be considered as a measure of variability for each pH probe/meter, because both samples were titrated to the same target pH with different titrants.
pH measurement comparison.
Based on the desire to obtain a clean data set for the BLA, a repeat LpH VC study was executed with Titrant A vs Titrant B using meter/probe #4 from the commercial manufacturing site. Comparable and robust LRFs were obtained (Table IV).
Repeat Viral Clearance Study: Titrant A vs Titrant B
Based on the results from the development study and the repeat study as well as discussions with the manufacturing network, the following conclusions were made:
pH measurements for the original VC study at Lab 1 were significantly different from the measurements obtained at Lab 2.
There were ultimately no viral safety concerns for the clinical batch manufactured in the Pilot Plant because worst-case higher titrant volumes were added (refer to Table I) to adjust the pH. From a product quality perspective, there were also no concerns, because no significant increases in aggregate levels were observed.
Consistent pH measurement for low pH VI is critical to ensure that both product quality and safety are not compromised at commercial-scale manufacturing.
Understanding of pH meter/probe differences between lab-scale, contract research organizations (CROs), and manufacturing is critical to ensure scale-down models are truly representative of commercial-scale manufacturing.
Alignment of pH meters/probes across multiple commercial manufacturing facilities can be challenging but extremely beneficial.
Application of Bracketing to Low pH VI (John Ruppino and John Mattila; Regeneron)
Low pH VI of recombinant proteins is typically evaluated under worst-case conditions of maximum manufacturing pH and minimum manufacturing hold duration. Acidic conditions below pH 3.6 provide effective and rapid enveloped virus inactivation. However, acid labile biotherapeutics may degrade rapidly under such conditions and require careful balance of VI and product stability. We evaluated low pH VI of a recombinant protein over a range of pH to assess process robustness and inform measurement tolerance to determine whether a bracketing approach could be applied to low pH VI above pH 3.60.
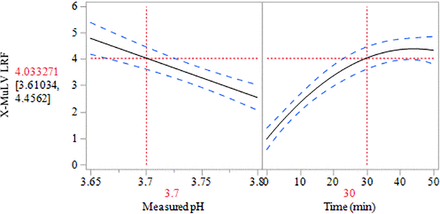
This prospective VI study included duplicate tests of an enveloped virus, X-MuLV, at three pH targets above pH 3.60. Inactivation kinetics were assessed by measuring infectivity periodically during a 50 min hold at each condition. Rate of inactivation was modeled as a function of pH and time, as shown in Figure 3. A restricted maximum likelihood model (REML) was generated based on measured pH during the study (3.66 to 3.80) and time (0 to 50 min), as shown in eq 1.
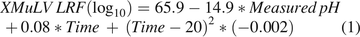 (1)
(1)
Low pH viral inactivation X-MuLV log reduction factor (LRF) modeled versus both measured pH and time (min). X-MuLV, xenotropic murine leukemia virus.
The model demonstrates that for this recombinant protein program, X-MuLV LRF shows a dependence on both measured pH and time at a 0.05 significance level. The model accounts for 85% of the variation in the data set (R2 = 0.85) with a root mean square error (RMSE) of 0.61 log10.
Based on the model, a ± 0.03 shift in measured pH, equivalent to probe measurement tolerance, is predicted to correspond to a change of ± 0.45 LRF at the 30 min time point. As such, there is a substantial effect of pH variation on X-MuLV inactivation. Furthermore, the model shows that X-MuLV clearance may be < 4 LRF when operating at pH > 3.70, indicating that consistent, effective clearance is not ensured in that pH range during a 30 min hold. The model predicts that operating at a maximum pH of 3.65 is likely to result in consistent, effective X-MuLV clearance of > 4 LRF as the model 0.05 significance level is above the 4 LRF threshold at pH 3.65.
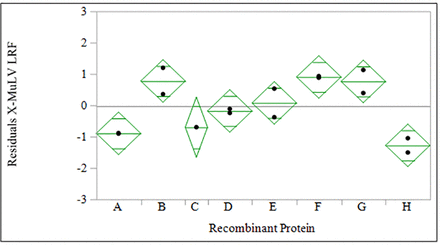
The prediction expression was used to predict X-MuLV LRF based on pH and time for eight additional recombinant protein programs not used in model generation. The differences in LRF between actual and predicted were compared to inform whether this model expression is applicable for a range of recombinant products, as shown in Figure 4. Across these eight recombinant protein programs, all have a mean actual LRF within 1 log10 of the predicted value, although five of the eight showing a nonzero residual (i.e., actual − predicted) at the 0.05 significance level. This indicates that the original model expression shows prediction power, and additional variability remains between processes that could influence X-MuLV LRF. Possible factors that could potentially influence X-MuLV inactivation, yet were not included in the prospective study design, include nonlinear response to pH, measurement tolerance, virus stock variability, and load conductivity, among others.
Plot of residual (actual − predicted) X-MuLV LRF for low pH viral inactivation processes not used in model generation shows nonzero residual for five of eight Regeneron recombinant proteins at a 0.05 significance level. LRF, log reduction factor; X-MuLV, xenotropic murine leukemia virus.
Data generated from a prospective low pH VI study demonstrate that a bracketed approach may not be applicable within this low pH VI dataset at pH > 3.70, as effective inactivation of a model retrovirus, X-MuLV, is not ensured under the study test conditions. The data instead supports a pH target of 3.65 that can balance effective VI with desired product stability. The model prediction expression that was applied to a collection of other recombinant protein processes showed prediction power, but some process variability remained unexplained by the model. Further characterization is ongoing to understand the presence and impact of additional factors on X-MuLV inactivation above pH 3.6.
Virus Clearance by Detergent-Dependent On-Column Inactivation (Matthias Kron; Rentschler Biopharma SE)
Virus inactivation in batch mode is the common procedure for most mAb platform processes. Among those approaches, virus inactivation by low pH incubation or by chemical inactivation using solvent/(eco-friendly) detergent (1), isopropanol, or benzonase treatment is commonly used. The absolute pH value or the effective concentration of the used chemical as well as incubation time and temperature are typical critical process parameters affecting the inactivation efficiency. Beside some challenges, for example, the subsequent removal of an added chemical (e.g., a detergent) or the technical hurdles for implementation of a batch-mode unit operation into a continuous process due to a lack of commercially available GMP production systems (2, 3), protein stability in solution toward acidic pH conditions or chemical treatment can be a major issue. Thus, a batch-mode approach cannot be applied for sensitive molecules, as it results in a loss of the bioactivity or a compromise of the protein structure. Due to product-related restrictions, a more demanding approach for robust virus inactivation is inevitable to ensure the viral safety of the process step.
One efficient strategy is the integration of virus inactivation into a chromatographic step (4, 5). On-column inactivation using a detergent-containing wash buffer offers various advantages. An immobilized protein, for example, bound to an affinity ligand, might be stabilized in its structure and withstand conditions that would otherwise compromise its structure in solution. By defining the flow rate or implementing a pause in the chromatography method, the incubation time as one critical process parameter is easily controlled. Operator-dependent process variability regarding added detergent amount and homogenous mixing is eliminated by a well-controlled buffer release procedure before the process. This approach allowing omission of a separate unit operation is also feasible for implementation into a continuous process without the need for further specialized equipment. The used detergent is immediately removed by passing by the resin-bound product and by subsequent wash steps. Therefore, no separate unit operation, for example, a tangential flow filtration, is required and the obtained chromatography eluate may contain only minimal trace amounts of detergent.
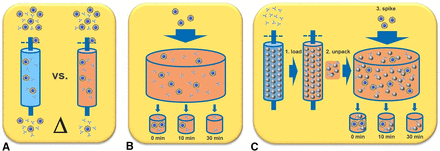
However, the design of a virus clearance study demonstrating the virus inactivation capacity of such an approach is more complex compared with a batch mode. According to the relevant guideline ICH Q5A “the possible mechanism of loss of viral infectivity should be described with regard to whether it is due to inactivation or removal”, and orthogonality of separation mechanisms between individual process steps claimed for virus removal has to be shown. Because the chromatography step itself might contribute to virus removal by physical separation mechanisms, for example, during column loading, washing, or elution, a simple study design validating the log reduction value (LRV) between virus-spiked load and remaining virus particles in the obtained eluate on the whole leaves a mechanistic black box in between and does not fulfill the guideline requirements. Therefore, more sophisticated validation designs are required to reveal the exact contribution of mechanisms to total virus removal. For this purpose, three possible approaches are suggested, as shown in Figure 5, which might be applicable under certain prerequisites and are described in the following.
Different virus clearance study designs for on-column inactivation. A) delta approach: performing two runs with or without detergent-containing buffers. B) simplified batch mode: adding virus-spike to an antibody/detergent solution with theoretically calculated effective concentrations. C) loaded-resin approach: using unpacked, product-loaded, and detergent-equilibrated resin bead slurry for a virus spike in batch mode.
1. Delta Determination
In this validation approach, the chromatography step is performed according to the defined process parameters with a validated scale-down model. The virus-spiked load material is applied on the column and the LRV is determined by detecting virus particles in the obtained eluate fraction. In a second run, the chromatography is performed analogously but without the detergent in the respective buffer. Thereby, the absolute LRV proportion of virus removal/inactivation mediated by the detergent in the first run is determined. Additional mechanistic insights can be gained by using complementary virus detection methods (TCID50 vs. quantitative PCR [qPCR]), discriminating between inactivated and active virus particles (or genomic remnants, respectively), and by the analysis of flow-through, wash, and regeneration fractions. This approach exactly reflects the process conditions happening on the column. However, the real inactivation potential of the detergent-containing buffer might be underestimated if the virus removal capacity of the chromatography step alone by physical separation mechanisms is already high and consequently the resulting delta is low. In addition, following inactivation kinetics is laborious and requires numerous runs. Therefore, this approach might only be feasible if the overall virus removal capacity of the process does not fully rely on the contribution of the detergent step or if the chromatography step itself has a low contribution to virus clearance.
2. Simplified Batch Mode
In this easy approach, virus-spike is added to an antibody/detergent solution with theoretically calculated effective concentrations present on the column. Optionally, resin beads or ligands can be added. Inactivation kinetics are studied by multiple sample drawing, whereas the full inactivation potential of the detergent is exploited. However, because the actual process conditions are not exactly reflected and the virus might be more susceptible to detergent inactivation, this approach is appropriate for feasibility studies. Worth mentioning is that a similar approach for an orphan drug was accepted for marketing authorization application by the European Medicines Agency (EMA) in 2017 in which data excluded a stabilizing effect of the resin on the virus (6).
3. Loaded Resin Batch Mode
This approach intends to mimic the actual process conditions (concentration ratios and molecule/bead interactions) more precisely, combining with the advantages of the simplified batch mode. Therefore, the chromatography step is regularly performed (without virus spike) and stopped when the product-loaded column is fully equilibrated in the detergent-containing buffer. At this stage, the column is unpacked, and the resin slurry is transferred into an incubation vessel where the virus spike is added to start the incubation period. This approach requires a more labor-intensive experimental setup and possibly pretests or a special sample preparation for the virus detection assay.
In summary, the loaded resin batch-mode approach is generally recommended as it allows claiming of the full removal/inactivation capacity of both the chromatography step and the detergent-mediated activation, and a broader acceptance by the authorities can be expected.
Understanding Solvent/Detergent VI Used in Recombinant Enzyme Purification Process (Junfen Ma, Skaison Kim, Fang Liu, Cecilie Klausen, and Johanna Kindermann; Takeda)
VI is one of the orthogonal methods used for VC in the recombinant protein purification process. There are different methods of VI, including low pH treatment, detergent, or combined solvent and detergent, and so forth. Solvent/detergent (S/D) VI has been widely used for plasma-derived products for >30 years as a robust VI method. It uses a combination of solvent and nonionic detergent to destroy enveloped viruses. The commonly used solvent is Tri-n-butyl phosphate (TnBP), and the nonionic detergents can be sodium cholate, polysorbate 20 (PS20), polysorbate 80 (PS80), and Triton X-100.
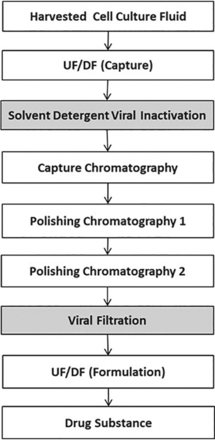
In this work, the S/D VI is used in the recombinant enzyme replacement therapeutic purification process. As shown in Figure 6, VI and viral filtration are dedicated VC steps in the recombinant enzyme purification process. Chromatography steps are assessed for VC if needed.
Recombinant enzyme purification process. UF/DF, ultrafiltration/diafiltration.
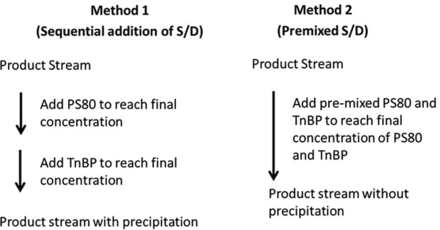
As part of implementation of S/D VI in the manufacturing process, one of the focus areas was how to add solvent and detergent into the enzyme process stream to avoid potential precipitation. Two methods were evaluated for addition of S/D into the product stream, as shown in Figure 7. In the first method, PS80 and TnBP were added to the product stream in a sequential manner to reach the final concentration. In the second method, the premixed PS80 and TnBP were added to the product stream to reach the final concentration of both components. The experimental details and results of comparing these two methods are summarized in Table V. Premixed S/D stock addition significantly reduces cloudiness during VI if the S/D is added to the harvest material. However, for the cleaner start material (buffer and purified drug substance), both the premixed method and the sequential addition method work well without generating cloudiness.
Two methods used for addition of solvent/detergent into the product stream. PS80, polysorbate 80; S/D, solvent/detergent; TnBP, tri-n-butyl phosphate.
Summary of Comparison of Two Solvent/Detergent Addition Methods
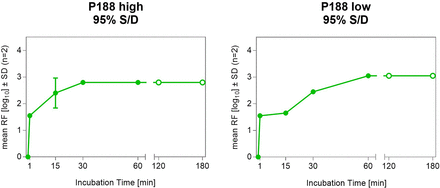
The other focus area was to understand if any cell culture media component, for example, Poloxamer 188 (P188), potentially impacts the kinetics of the VI and the overall LRF. P188 is used in the cell culture process to protect cells from shear and maintain cell viability. Based on the data collected (not shown here), P188 is co-concentrated with the enzyme product during the initial ultrafiltration/diafiltration (UFDF) capture step as shown in Figure 6. The impact of the P188 concentration was investigated on the X-MuLV inactivation by S/D with PS80 and TnBP for a duration of up to 3 h. Two P188 concentrations were studied, one at approximately 20 g/L (low) and the other at approximately 56 g/L (high). The tested concentration of PS80 and TnBP was 95% of the target concentration used in the manufacturing process. As shown in Figure 8, both VI kinetics and actual LRF are very similar between the two concentration levels of P188 evaluated.
Viral inactivation of X-MuLV with solvent/detergent at different concentrations of Poloxamer 188. P188, Poloxamer 188; RF, reduction factor; S/D, solvent/detergent; X-MuLV, xenotropic murine leukemia virus.
To summarize, S/D VI was demonstrated as a robust and dedicated VC step in the biopharmaceutical purification process. In the implementation of the S/D inactivation step, premixed S/D stock addition significantly reduces cloudiness. In addition, poloxamer, as a shear protectant used in the cell culture, does not impact the effectiveness of the S/D VI.
Detergent-Based Virus Inactivation: Development of an Alternative for Triton X-100 (Johanna Kindermann, Jean-Baptiste Farcet, and Thomas R. Kreil; Takeda)
After the transmissions of viruses through plasma derivatives was recognized in the early 1980s, first VI and later removal steps were implemented into their manufacturing processes. These measures have kept plasma derivatives safe ever since, also against more recently emerging viruses. Given the success of these interventions, they also have been embedded into the manufacturing processes for cell-derived biological medicinal products.
The most effective inactivation process for lipid-enveloped viruses is treatment by detergents, or combinations of solvents and detergents, and thus these processes have been adopted almost universally. One of the most widely used detergents, Triton X-100 (TX-100), recently has raised environmental concerns because one of its degradation products possesses hormone-like (estrogen-mimetic) activity that may act on wildlife animals. As a consequence, use of the chemical in the European Union will ultimately be prohibited (7).
Thus, a study was conducted to develop an environmentally friendly and fully functional alternative detergent for biotechnological uses. Following the desire for minimal differences between TX-100 and the potential replacement candidates as design principle, the new molecule should ideally be a nonionic and polyethylene glycol (PEG)-based detergent with a low CMC. Unlike TX-100, however, it should not contain the phenol entity, as this chemical group is revealed when the molecule is metabolized in the environment to become an estrogen receptor-binding pseudohormone. Several of the commercially available detergents that have been suggested as TX-100 replacement products exhibit considerable structural difference. An investigation comparing potential replacement candidates, that is, PS20, Triton X-100 reduced, and Brij C10, an n-hexadecyl polyethoxylated compound, with the traditionally used TX-100 was performed. The results of the initial screening pointed out that PS20 poorly inactivated viruses, whereas TX-100 reduced and Brij C10 had acceptable VI properties in standard temperature processes but showed only moderate inactivation when tested under cold conditions. The limited number of commercially available detergents that met the predefined structural requirements resulted in rather narrow screening possibilities. As a consequence, an in-house synthetized new detergent library was established, with the intention of deleting the phenol moiety of TX-100 but retaining a similar core structure. One of these novel detergents, Nereid, was identified as an ideal match. As part of a three-component S/D mixture, the performance of Nereid was fully equivalent to those of TX-100, TX-100 reduced, and Brij C10 in the plasma-derivative model matrix. Notably, the virus-inactivating properties of Nereid were also similar to that of TX-100 in the recombinant process material under unfavorable cold conditions.
Over the last decades, biotechnological production processes have substantiated the effectiveness of the TX-100/TnBP/PS80 combination against enveloped viruses. However, to the best of our knowledge, scientific evidence explaining why exactly these three compounds have to be combined for optimum antiviral performance has never been presented. Thus, it was decided to investigate the virus-reducing properties of TX-100 if used as a single detergent, and to compare its performance with that of the most promising surrogate candidates TX-100 reduced and Nereid. Although a moderate or no inactivation under cold treatment conditions was obtained for TX-100 reduced, the effectiveness of TX-100 and Nereid was fully maintained even at these “worst-case” conditions. These data suggested that even the use of a single detergent can result in robust and effective virus inactivation, potentially paving the way toward a significant reduction in manufacturing process complexity.
In conclusion, a newly synthesized compound, Nereid, seems to fully satisfy all requirements and thus can be an ideal Triton X-100 replacement for virus inactivation in biopharmaceutical manufacturing.
Summary
Alternative eco-friendly and cost-effective detergents were demonstrated to provide robust VI in the biologics manufacturing processes. These include sugar-based detergents and TX-100 analogs, for example, Nereid, a nonionic and PEG-based detergent formed by deleting the phenol moiety of TX-100 but retaining a similar core structure.
For low pH VI, consistent pH measurement is critical to ensure product quality and product safety. Understanding of pH meter/probe differences between lab-scale, CROs for virus spike study, and manufacturing is critical to ensure scale-down models are truly representative of manufacturing. Alignment of pH meters/probes across multiple labs and manufacturing facilities can be challenging but extremely beneficial. In addition, it was demonstrated that a bracketed approach may not be applicable at pH > 3.70. Instead, a pH target of 3.65 can balance effective VI with desired product stability, suitable for acid labile biotherapeutics.
For S/D VI used in the recombinant enzyme process, premixed S/D stock addition significantly reduces cloudiness. In addition, poloxamer, as a shear protectant used in the cell culture, does not impact the effectiveness of the S/D VI.
On-column VI using a detergent-containing wash buffer offers the benefit of overcoming the product stability-related restriction from the batch-mode operation as well as eliminating the unit operation of VI itself to simplify the overall downstream process. However, the VC validation design is required to reveal the exact contributions of the mechanisms (chromatography or VI) to the total viral removal. For this purpose, three possible approaches were suggested, including delta determination, simplified batch mode, and loaded resin batch mode. The loaded resin batch-mode approach is generally recommended as it allows claiming of the full removal/inactivation capacity of both the chromatography step and the detergent-mediated activation, and a broader acceptance by the authorities could be expected.
Conflict of Interest Declaration
The author declares that they have no competing interests.
- © PDA, Inc. 2022