Abstract
Leachables are quantified and identified to enable their quantitative toxicological safety risk assessment (qTSRA). The leachable’s reported concentration and identity must meet certain quality expectations to be suitable for qTSRA. In this Correspondence, the author considers accuracy and protectiveness as competing key quality attributes and suggests that protectiveness is the proper quality attribute for qTSRA, as qTSRA is based on the foundation that a leachable’s potential adverse effect on patient health and safety must not be underestimated. Considering this conclusion, means of making concentration estimates and proposed identities protective are discussed.
Introduction
Packaged drug products may contain impurities, termed leachables, which are derived from the drug product’s packaging or manufacturing equipment. Furthermore, substances can be leached from medical devices during their clinical use. In any case, leachables (and extractables as potential leachables) are identified and quantitated to enable their toxicological safety risk assessment, as the leachable’s concentration is a critical input into calculating patient exposure, and the leachable’s identity is used to establish its inherent toxicity (e.g., permissible exposure).
It is frequently the case that extractables and leachables analysis is an exercise in non-targeted (or screening) analysis, NTA, as the purpose of the analysis is to characterize a test sample (extract, leachate, or drug product) for unspecified analytes, where characterization is understood to mean (a) produce a recognizable response for all analytes present in the sample above an established reporting threshold and (b) use the responses, and the information contained therein, to estimate the analytes’ concentrations in the sample and establish their likely identity. This contrasts with target analysis (TA), where the analytes are specified upfront (no identification necessary) and highly accurate concentrations are obtained via calibrated response functions.
Using a leachable’s identity and estimated concentration, obtained via NTA, to assess whether a patients’ exposure to that leachable during a clinical therapy adversely affects patient health and safety means that the leachable’s reported concentration and identity must meet certain quality expectations. The objective of this Correspondence is to contrast the quality expectations of accuracy (reporting the true value) and protectiveness (reporting a worst-case value).
Accuracy versus Protection
According to USP <1225> (1), the accuracy of an analytical procedure is the closeness of the test results obtained by that procedure to the true values. When considering quantitation, the true value is the analyte’s true (actual) concentration in a sample. Although an accuracy of 100% (reported concentration = true concentration) is highly desirable, one recognizes the variation and uncertainty that is inherent in applying an analytical method for quantitation and thus one understands accuracy in the context of an acceptable range. Thus, for example, an analytical method could be established to be quantitative if the accuracy falls within a range of 90% to 110% of the true value.
Considering identification, the true value is the analyte’s actual identity. Unlike quantitation, where accuracy could be expressed as an acceptable range of recoveries, in identification the analytical outcome (the reported identity) is either correct (accurate), meaning the reported identity is the actual identity, or incorrect (inaccurate), meaning the reported identity is not the actual identity.
The concept of protectiveness is understood considering the use of quantitative and identification information obtained in NTA of extractables and leachables. Specifically, the most common use of extractables and leachables information is quantitative toxicological safety risk assessment (qTSRA), where the purpose of qTSRA is to establish whether the extractable or leachable poses a threat to patient health and safety. That is, qTSRA is performed to protect a patient from unsafe extractables and leachables.
For qTSRA to be protective, the analytical data upon which it is based must be protective. This means that the data must be such that patient exposure to a leachable (linked to the leachable’s reported concentration) is not underestimated, as underestimating patient exposure produces a qTSRA that understates the patient’s safety risk. Moreover, the inherent toxicity of the leachable (linked to its identity) cannot be underestimated, as underestimating the leachable’s inherent toxicity produces a qTSRA that understates the patient’s safety risk.
Quantitation
Most practitioners, when asked to specify the goal of quantitation, would respond that the goal is to produce an accurate result where the calculated concentration is the same as the analyte’s true value, within an acceptable range of deviation. Although this is a desirable outcome to be sure, it may not be the optimum or desired response in the context of qTSRA. As the goal of qTSRA is to establish whether a patient’s health is adversely affected by exposure to leachables, it is imperative that the patient’s exposure to leachables, and thus the leachables’ calculated concentrations, is not underestimated. If a leachable’s concentration is either accurately estimated or overestimated (its reported value is equal to or greater than its true value), then patient exposure to the leachable will be either properly estimated or overestimated. In this circumstance, the reported concentration is said to be protective, as use of the reported concentration in the qTSRA will ensure that the leachable’s potential adverse effect on patient safety is not underestimated. If the leachable’s reported concentration is underestimated (the reported concentration is lower than its true value), then patient exposure to that leachable will also be underestimated in qTSRA, and it is possible that the qTSRA could conclude that a leachable was safe when in fact the leachable could adversely affect patient health. In such a circumstance, the reported concentration is not protective, as the reported concentration does not ensure patient safety.
In this context, these potentially conflicting goals of leachables quantitation can be defied as follows:
Accurate: The reported concentration is the same as the true value (within a range of acceptable deviation)
Protective: The reported concentration is not less than the true value (meaning that the calculated concentration is equal to or greater than the true value).
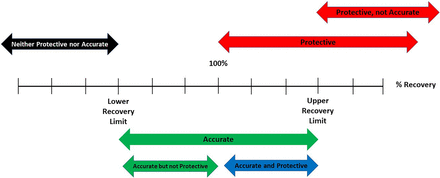
The juxtaposition between accurate and protective quantitation is illustrated in Figure 1, which establishes a continuum of percent recoveries as an indicator of accuracy, and highlights 100% recovery (true value) and the minimum and maximum acceptable % recoveries for the reported concentration to be considered accurate. In this context, the term is taken as:
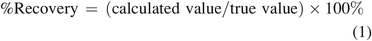
The continuum of recoveries, illustrating the concepts of accuracy and protection. The continuum includes the critical values of 100% recovery (true value), the Lower Recovery Limit (the lowest recovery for accurate quantitation), and the Upper Recovery Limit (the highest recovery for accurate quantitation). The continuum illustrates how quantitation can be accurate but not protective, protective but not accurate, both protective and accurate, and neither accurate not protective.
Across this continuum, one notes regions where the reported concentration is:
neither accurate nor protective,
accurate but not protective,
accurate and protective, and
protective but not accurate.
For example, consider a situation in which an analytical method is said to be accurate if recoveries range from 80%–120%. If:
the recovery for an analyte is 70%, the method is neither accurate nor protective for that analyte;
the recovery for an analyte is 90%, the method is accurate but not protective;
the recovery for an analyte was 110%, the method is both accurate and protective;
the recovery for an analyte is 130%, the method is protective but not accurate.
A critical distinction between TA and NTA is the acceptable % recovery range for the test method to be considered accurate. In TA, highly accurate quantitation is achieved by validated methods supported by calibration curves generated for the target analyte(s). As such, quantitation by TA is characterized by acceptable accuracy ranges that are quite tight; for example, 90%–110%. This is contrasted to quantitation in NTA, which typically is achieved by means other than calibration curves and validated methods and which have acceptable accuracy ranges that are much broader (e.g., 50%–200% [2], appropriate for trace analysis).
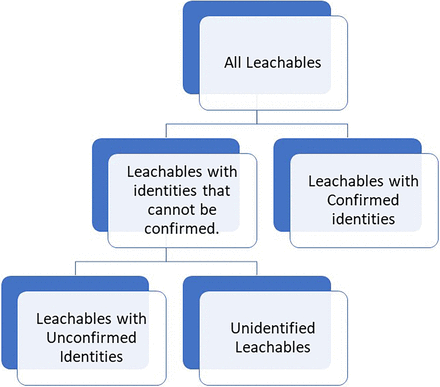
The distinction between being accurate or protective is important in leachables NTA given the various means by which quantitation can be achieved in NTA. To understand these means and their accuracy/protection implications, the population of all potential extractables or leachables is broken up into two groups, those compounds that can be identified to a confirmed status and those that cannot (see Figure 2). This distinction is important, as it differentiates compounds that can be quantified with higher accuracy (those compounds with confirmed identities) from those compounds that will be quantified with lower accuracies (those compounds for which confirmed identities cannot be obtained). This distinction leverages the circumstance that a confirmed identity can only be secured by matching critical features of a compound’s analytical response (e.g., retention time and mass spectrum) with the same critical features for a reference standard analyzed via the analytical method in question (see USP <1663> [3]). This distinction becomes important for quantitation, as one of the critical features that is secured for a reference standard is its response factor, or in the case in which an internal standard is used as a reference, the relative response factor (RRF). The internal standard is added to every analyzed sample (extracts and standards) to account for sources of analytical variation such as injection-to-injection variation. It is this RRF that allows compounds with confirmed identities to be quantified with higher accuracy. This higher accuracy results from the circumstance that the compound is quantitated knowing its own response characteristics. In essence, if a compound’s response function (relationship between concentration and response) is linear, the RRF is the slope of the response function. Practically speaking, in the linear range of a response function, use of the RRF for quantitation is essentially as accurate as the use of a calibration curve.
Identification categories as the key driver of quantitation. Discovered compounds are placed into identification categories, based on the certainty of the identification, as a prerequisite to quantitation. The means by which compounds are quantified and the degree to which a calculated concentration is accurate and/or protective depends on the compound’s identification classification, with the most accurate quantitation’s being produced for compounds with confirmed identities and established RRF values. RRF, relative response factor.
It is intuitive that use of a confirmed analyte’s own RRF for quantitation will produce an accurate reported result. However, as recoveries of the compound may vary somewhat within and between analytical runs, possibly the reported result would not be rigorously protective (i.e., a recovery of <100% that still falls in the acceptable accuracy range). Nevertheless, as recoveries obtained with a compound’s own RRF will not be significantly <100% (e.g., a recovery of 85% is not significantly lower than 100%), the degree of underestimation will be sufficiently low that for all practical purposes the reported results are adequately protective. However, to be truly protective, an underestimation factor can be applied, described as follows.
Thus, when a leachable is quantitated using its own RRF value, the calculated concentration is both accurate and generally protective. However, to ensure that a concentration secured via a matched RRF is fully protective, one must consider the range of accuracy (recoveries) that can be secured with matched RRF values. If it is proposed that a concentration secured by matched RRF has an accuracy of between 85% and 115%, then a concentration secured by matched RRF becomes fully protective when the calculated concentration is adjusted using an underestimation factor, which in this case is 100%/85% = 1.18.
It is obvious that securing a confirmed identity for all extractables and leachables is the most desirable outcome; however, the practical reality is that there are many extractables and leachables for which a reference standard is not commercially available or which have not been produced (e.g., synthesized or isolated/purified) and qualified by individual laboratories as reference standards. These extractables cannot be identified to the confirmed level (and cannot be quantified with their own RRF) for the simple reason that no reference standard exists. The lack of a reference standard means these compounds cannot be quantified based on their own response characteristics and therefore they must be quantified based on the response characteristics of a surrogate compound or surrogate compounds. This circumstance means that compounds without confirmed identities will be quantified with lesser accuracy versus compounds with confirmed identities, as there is some level of inaccuracy inherent in quantification via a surrogate (as no match between an analyte and a surrogate is perfect for all analytes).
In theory, compounds without confirmed identities can be further subdivided into compounds for which an identity can be proposed (unconfirmed identities) and compounds for which identities cannot be proposed (unidentified). This distinction is potentially important considering the premise that a compound can be quantified with some higher degree of accuracy if it can be structurally or functionally matched to a surrogate standard whose response characteristics are similar to those of the compound of interest. By using the RRF from a closely matched and similarly responding surrogate for quantitation, a compound whose identity has been proposed but unconfirmed can be quantified with an accuracy that is only somewhat less than the accuracy that could be obtained if that compound had been identified to a confirmed level and quantified with its own RRF. If it is proposed that a concentration secured by a matched surrogate RRF has an accuracy of between 50% and 200%, then a concentration secured by matched surrogate RRF becomes fully protective when the calculated concentration is adjusted using an uncertainty (underestimation) factor of 100%/50% = 2.0.
In practice, the ability to match compounds’ responses via their structures is a goal but not a reality in extractables and leachables in NTA. Although considerable attention has been given to this concept, the only published study that examined this premise (4) concluded that “retention time and/or structure matching is not a solution to the challenge of providing quantitative data when screening for extractables and leachables”.
In the circumstance in which identity cannot be used to link an analyte with a similarly responding surrogate, compounds with unconfirmed identities and unidentified compounds are “quantified” based on linking these compounds to a single surrogate standard. Specifically, such compounds are quantified using the single surrogate’s RRF. As one suspects, linking all such compounds to a single surrogate is accurate only in the case in which all compounds respond similarly. It is well-established that this is not the case for extractables and leachables, whose RRF values vary widely in both gas and liquid chromatography with mass spectrometric detection (GC/MS and LC/MS). Thus, quantitation with an unmatched single surrogate standard is likely to be inaccurate and potentially unprotective.
Through a judicious choice of the single surrogate standard, protectiveness can be optimized somewhat. For example, it has been proposed that the proper surrogate standard is a compound whose RRF value is the median of the distribution of response factors for reference standards (5, 6). If the population of reference standards is sufficiently large and chemically diverse, then the RRFmedian of the population of the reference standards will be the same RRFmedian of the larger population of all extractables and leachables.
If the RRFmedian is used for quantitation, then quantitation is protective for 50% of the entire population of extractables and leachables. The fraction of the population for which this approach is accurate depends on the actual distribution of the RRF values and the criterion used to define accuracy. The tighter and more well-behaved the distribution of RRF values, the greater the fraction of extractables and leachables that will be accurately quantified. The broader the range of acceptable % recoveries established to define “accurate”, the greater the fraction of extractables and leachables that will be accurately quantified.
It is obvious that the 50% protection afforded by use of the RRFmedian is not adequate for qTSRA. Although it is unclear what an acceptable level of protection is, it is surely also obvious that 100% protection (where the reported concentration is always equal to or greater than its true value) is not a realistic expectation. Without taking a stand on what the acceptable level of protection is, this author nevertheless notes that protectiveness of quantities secured via RRFmedian can be increased by applying an uncertainty factor (UF) to the calculated concentration, similar in concept to the way the UF is used to adjust the analytical evaluation threshold (AET) for response variation (e.g., ISO 10,993:18(2020) [7]). However, whereas the UF is used to decrease the AET and make it more protective, the UF is used to increase the calculated concentration and make it more protective (eq 2).
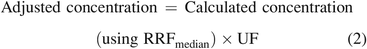
The increase in protection secured by such an adjustment depends on the distribution of RRF values. As UF is generally calculated based on the relative standard deviation (RSD) of the RRF population (meaning that the UF is a one standard deviation adjustment), if the distribution of RRF values were normal, then the adjusted concentration would be protective for 84.1% of all extractables and leachables. However, it has been established that RRF distributions for GC/MS and especially LC/MS are not normal and thus specifying the degree of protectiveness for these NTA methods is less straightforward. Nevertheless, based on published RRF distributions for GC/MS (8), this author estimates that a UF adjustment of a factor of 2, in line with RSDs of approximately 50%, would be approximately 80%–85% protective. In other words, use of RRFmedian and a UF = 2 would be protective by GC/MS for 80%–85% of all possible leachables. Given the much greater RRF variation and the more poorly defined distribution of RRF values for LC/MS (8, 9), this author cannot estimate how protective an LC/MS method would be when a UF is applied to the concentration calculated using RRFmedian, although use of a minimum value of 5 for the UF would be protective for 70% (or more) for all leachables.
Although mathematical means of making reported concentrations more protective for compounds whose identities cannot be confirmed have been discussed, it is this author’s opinion that the only means for properly and correctly producing an accurate and protective concentration estimate for a specific leachable is to secure a reference standard for that leachable, elevating that leachable to a confirmed identity and securing the compound’s own RRF to be used in quantitation.
Identification
Most practitioners, when asked to specify the goal of identification, would respond that the goal is to propose an identity for a compound that matches its true identity. Although this is a desirable outcome to be sure, it may not be possible to achieve this outcome in all circumstances. As the goal of qTSRA is to establish whether a patient’s health is adversely affected by exposure to leachables, it is imperative that the patient’s safety risk due to exposure to leachables is not underestimated. Such an underestimation could occur if a leachable was misidentified as a compound that is inherently more safe (potentially less toxic) than the actual compound.
In this context, these potentially conflicting goals of leachables identification can be defied as follows:
Accurate: The reported identity is the true identity
Protective: The reported identity is the possible identity that poses the greatest risk to patient safety.
Clearly a confirmed identity meets these goals and is fully protective and accurate, as the possibility that a confirmed identity is incorrect is vanishingly low. USP <1663> describes a confident identity as an identity “bolstered by additional and sufficient confirmatory information to preclude all but the most closely related structures” (3). Thus, the likelihood that a confident identity is incorrect is low and likely a confident identity, based on multiple corroborating pieces of evidence, is protective and accurate. However, a tentative identification is, by definition, an identification that could be incorrect, as the identification is based on only a single piece of evidence (e.g., a match to a database). As clearly an incorrect identity leads to a flawed qTSRA, there is the chance that a tentative identity is not protective. This occurs when the actual compound’s toxicity profile is worse than the toxicity profile of the compound it has been misidentified as being. Thus, a tentative identity is protective only in the case that the reported identity represents the candidate identity with the worst toxicity profile. Therefore, the goal of protective identification applied to tentative identities could be to report that candidate compound with the poorest safety profile (greatest intrinsic toxicity, lowest tolerable daily intake). For example, consider a compound whose tentative identity is based on mass spectral matching to a database. Furthermore, consider the case in which the spectral matching produces three possible identities, all with comparable match factors. Typically, this compound would be reported as being the candidate with the best match (the primary candidate) and nothing is reported about the possible alternatives. Alternatively, all three possible identities could be reported and the qTSRA could at least consider the candidate identity with the worst toxicity profile. Subjecting the most toxic identity to qTSRA will ensure that the compound’s toxicity profile is not underestimated.
With this in mind, the protective practice for reporting lower confidence tentative identities involves reporting the primary candidate and the other probable candidate identities for the compound of interest. Although it is proper to report which of the three candidates is the most probable (primary) identity, the whole point in reporting the other viable candidates is to admit that “there is a certain amount of uncertainty associated with this identity and this most probable identity might not be the true identity”.
One immediate response to the above is to lament that “now we have more work for tentatively identified compounds because we have to assess multiple compounds instead of one”. Although this is literally true, performing full assessment on all possible candidates (to establish which candidate has the highest toxicity) is not the recommended practice. Rather, less labor-intensive approaches, such as QSAR analysis for alerting structures, would be a proper means of ranking the candidates in terms of their latent toxicity. Although it is the primary candidate that undergoes full qTSRA, the assessment should include at least some consideration of the candidate that is likely most toxic.
It is occasionally the case that a specific identity cannot even be tentatively proposed for a leachable but that the leachable’s likely general structural characteristics can be inferred from the available data. For example, a leachable might be partially identified as “a phthalate” or “an aromatic hydrocarbon”. In this case, a protective qTSRA could be performed for the partially identified leachable using the most toxic compound that fits in the leachable’s structural category. For example, if a leachable were identified as “a phthalate”, the protective qTSRA could be performed using di-(2-ethylhexyl) phthalate, DEHP, as arguably the most toxic compound that is “a phthalate”.
However, in many cases, such an approach is overly protective. Using the previous example, it should be obvious whether the partially identified compound is actually DEHP. If, in fact, it can be established that the compound cannot be DEHP, then the qTSRA is not performed using DEHP as the basis but rather is performed on the viable phthalate candidate that poses the greatest patient safety risk.
This process of establishing which is the proper surrogate for performing qTSRA using a partial identity is not necessarily straightforward and caution is advised when using this approach.
It is clear (is it not?) that qTSRA cannot be performed for an unidentified compound, as there is no basis for establishing the latent toxicity of the unidentified compound. Although arguably the ability to establish that the unidentified compound is not a substance of exceptionally high toxicity (e.g., cohort of concern) simplifies the safety assessment of unidentified compounds somewhat (by establishing a threshold for the compound’s latent toxicity), so doing is a small victory and is insufficient to enable qTSRA.
Although a means of making reported identities more protective for compounds whose identities cannot be confirmed has been discussed, it is this author’s opinion that the only means for properly and correctly producing an accurate and protective identity for a specific leachable is to secure a reference standard for that leachable, elevating that leachable to a confirmed identity and erasing all doubts whether the reported identity is correct.
The Downside of Excessive Protectives (Excessive Overestimation)
To this point, the effect of using a UF adjustment on protection has been considered. But what about the effect of UF adjustment on accuracy? Qualitatively, application of the UF adjustment will have a profound effect on the accuracy of individual compounds. Some compounds whose calculated concentrations were lower than the low accuracy bound (poor responders) will be elevated into the accuracy range once the UF is applied. Some compounds whose calculated concentrations were within the accuracy range will be moved above the range when the UF is applied. If the RRF distribution were normal, then the effect of adjusting with the UF would be that the number of compounds that are accurate would decrease, as a fewer number of lower responders become accurate and a greater number of higher responders become inaccurate (adjusted concentrations higher than the upper accuracy bound).
Thus, we see that the penalty for being protective on the quantitation side is that most analytes will have reported concentrations that are higher than (and in some cases much larger than) their true value. Therefore, likely in these cases a patient’s exposure to certain leachables will be significantly overestimated. On one hand this large overestimation is protective, as the patient safety effect of these leachables will not be underestimated. On the other hand, this large overestimation could be deceptive, if, for example, the leachable were deemed to be potentially unsafe due to an overly inflated patient exposure based on the overly inflated reported concentration. In this case, the downside of being protective would be the possible rejection of a medical item as being potentially unsafe due to leachables, when in fact the item is acceptably safe.
This same downside is applicable to identification. When multiple possible identities are reported for tentatively identified leachables and the qTSRA considers the toxicity potential of the most unsafe of the reported compounds, there is always the chance that in fact the leachable’s true identity is one of the toxicologically less risky alternatives. As was the case with quantitation, this outcome would be protective but deceptive, potentially suggesting that a medical item could be unsafe when in fact the item is acceptably safe. This is why the proposed approach is to subject the best (most likely, primary) identity to qTSRA but to also consider the toxicity of other viable candidates in the assessment.
The purpose of this discussion is not to discourage protective practices for quantitation and identification. Rather, the reader is cautioned to critically examine individual results obtained by application of a group-based protective strategy, looking for individual cases in which the general approach to being protective is so excessive that issues are created and not merely surfaced.
Conclusions
Quantitation and identification of leachables (and extractables as potential leachables) are necessary activities to enable qTSRA. Clearly, a leachable’s estimated concentration and proposed identity must meet certain quality expectations, the most obvious of which is that they be accurate. However, when this data is used to support qTSRA, it is arguably more important that these quantities be protective; that is, that the qTSRA performed with the data does not underestimate the patient’s safety risk. For estimated concentrations, a protective concentration is a concentration that is not lower than the true concentration. For proposed identities, a protective identity is that proposed identity with the greatest toxicological safety risk.
Means of producing protective concentration estimates and proposed identities were proposed in this Correspondence and are summarized in Tables I and II.
Strategies for Producing Protective Concentrations
Strategies for Producing Protective Identities
Conflict of Interest Declaration
The author has no conflicts of interest to disclose; however, the author notes his affiliation with a contract research organization (CRO) that provides extractables and leachables services to the pharmaceutical and medical device industries.
- © PDA, Inc. 2024