Introduction
It is fairly common knowledge in the pharmaceutical, biotechnology, and medical device industries that regulatory authorities expect good manufacturing practice (GMP) facilities to demonstrate that the biocides used in controlled environments are effective against environmental isolates on surfaces representative of the area in which they are used. This article will focus on the inherent challenges associated with aseptic manufacturer end user disinfectant efficacy coupon testing, aspects of which have been cited by various regulatory agencies during inspections. Germicide manufacturer disinfectant registration testing according to U.S. Environmental Protection Agency (EPA) test methods, American Organization of Analytical Chemists (AOAC) test methods, or European Norms (EN) possesses a different set of intrinsic challenges and is outside the scope of this article.
Disinfectant effectiveness studies are not routine and require an extensive amount of planning whether they are performed internally or by a contract lab. Due to the resources and time involved, it is especially disheartening when the results do not meet the established acceptance criteria. An investigation is required to determine what caused the failure in order to justify repeat testing. That said, finding the exact reason(s) that a biocide failed to show effectiveness against a particular organism or on a particular surface is challenging because it is often difficult to pinpoint the exact cause for the failure. The following examples of U.S. Food and Drug Administration (FDA) Form 483 citations show that regulators are looking closely at disinfectant effectiveness studies:
Results from the disinfectant efficacy studies also reported that challenges of contact time on surfaces mimicking flooring and front curtain surfaces found that the agent … was not effective (1).
Examination of the reports from the contractor found that the contact time challenge of … against Bacillus subtilis was effective on stainless steel surfaces. However, the contact time on the … surfaces for the same organisms was ineffective. There was no evidence to indicate the study was repeated (2).
Specifically, during the execution of the disinfectant efficacy study of … solutions against Gram Negative Organisms, Yeasts and Molds, the firm encountered a deviation when three surfaces did not meet the criteria for Aspergillus brasiliensis. The report referred to a deviation, noted “aberrant results,” and re-executed the study for the failing criteria. However, the deviation log failed to document the root cause of the initial failing results and scientific justification for invalidating the initial results (3).
There is no assurance that the disinfectant … is effective against mold, since it did not meet your established recovery rate acceptance criterion … (4).
In vitro testing to demonstrate effectiveness of disinfectants and sporicides, particularly those involving surface challenge, is inherently difficult to perform and presents numerous challenges. There are many factors that are critical to the study outcome, and if not carefully controlled then they will lead to failures. Numerous materials, extensive preparation, and significant technical resources are necessary to prepare and properly execute such a study. Failing or unexpected results compound a process that is already complex, time-consuming, and expensive. Being aware of potential issues up front and how to prevent or address them can make the process go smoother. This article highlights some of the critical steps during in vitro testing that present significant challenges when performing this testing and could lead to failures.
When a failure occurs, some may question whether or not the product is effective. A reputable biocide manufacturer performs extensive testing during development to determine the effectiveness and limitations of the product, and the claims on the label are the result of significant data development and review. Prior to marketing the product, extensive data packages demonstrating, among other things, chemical stability and efficacy under prescribed conditions (e.g., soil load, temperature, inoculum prep, specific organisms, and log reductions) are developed and reviewed by the governing regulatory body. For example, in the United States, this authority is the U.S. EPA, in Brazil the authority is National Health Surveillance Agency Brazil (ANVISA) and in the Netherlands it is Board for Authorization of Plant Protection Products and Biocides (Ctgb). Nearly every country has its own registration authority and most have a preferred testing scheme. It is these governing authorities that review the extensive data packages prepared by the manufacturers and that determine the appropriateness of marketing the specific product with specific label claims. While there is a possibility that products with borderline effectiveness are on the market, it is important to realize that there are other factors to investigate when a failure occurs.
The activity of a disinfectant can vary considerably from method to method even with the same product. Therefore, it is crucial that the method chosen be used throughout the disinfectant qualification study. Consistency when performing the study is critical. Once the appropriate method has been chosen, there are several parameters that must be closely controlled for consistent results. Any one of these parameters alone can be the root cause for a test failure; when several occur together, the likelihood of a failure is multiplied. These parameters can be divided into five key areas and are listed in Table I.
Most Common Causes for Failure
Log Reduction Acceptance Criteria
The acceptance criteria are often related to the level of bioburden found in the area being disinfected. It is important to consider that the bioburden level in an ISO Class 5 cleanroom, for instance, will be exceptionally low. Setting acceptance criteria requiring unusually high log reductions sets one up for failure without reason. The acceptance criteria for log reduction from a surface challenge test can vary between methods and regulatory agencies (Table II).
Acceptance Criteria (5–7)
General Causes
One of the most important steps prior to beginning a study is reading the biocide label and understanding the claims and limitations for each biocidal product. Knowledge of the broad spectrum efficacy of the biocide being tested is important in understanding the expected outcome. Careful examination of the manufacturer's label to determine which of the test microorganisms the biocide will be effective against, the preparation instructions, and the contact time will help to prevent development of a flawed protocol. If a disinfection program uses an inappropriate fungicidal or sporicidal agent, the risk of serious contamination events is greatly multiplied and efficacy may be impossible to demonstrate in the disinfectant qualification study.
It is essential that the correct challenge organisms are chosen for each chemistry type included in the study. For example, testing 70% isopropyl alcohol (IPA) or a quaternary ammonium compound–based disinfectant against bacterial spores will likely result in a failure. Not all active ingredients are effective against all types of organisms. However, formulation of the product does make a difference, and two products with the same active ingredient(s) may have different label claims.
Trending environmental monitoring data can be helpful, but only if it is used to make good decisions about how to control and maintain the critical environments. One such approach is to review product label claims against the isolates detected and identified in the areas to determine if they are well matched. For instance, Trichophyton mentagrophytes is the representative fungus required for an EPA fungicidal label claim. Performance against this organism does not necessarily mean the biocide will be effective against all fungi. T. mentagrophytes is much less resistant to many biocides than Aspergillus brasiliensis, which is a much greater challenge and is more likely to be found in a manufacturing environment. A sporicide may be more effective against resistant fungal spores.
Sometimes a contact time that is shorter than what is specified on the product label is desired by the user. Testing at a shorter contact time can be performed, and in some cases it may prove to be effective. However, if there is a failure at a contact time shorter than that recommended on the product label, it does not necessarily mean the biocide is ineffective, only that it is ineffective at the conditions being tested. Use of a shorter time than stipulated on the label is a valid justification for retesting in the event of a failure. That said, please keep in mind that the failure may also be due to other factors that will be explored further in this article.
If using a concentrated biocide, be mindful of the manufacturer's instructions for preparation and mixing. It is critical to measure volumes accurately when preparing the use-dilution. Incorrectly prepared use-dilutions may lead to results that can overestimate or underestimate the effectiveness of the product. Using the recommended use-dilution is also advisable because of potential surface deterioration or corrosion issues that may occur at higher use concentrations.
Inoculum
Properly preparing an inoculum suspension is critical to the success of any disinfectant qualification. Poor viability of bacterial suspensions and other issues ranging from extracellular debris, spore aggregation, contamination, and issues associated with drying the inoculum can lead to failures. Proper technique and knowledge of the organisms included in the study are of paramount importance. The growth and preparation of the challenge organism determines the physiological state of the cell, which has a direct influence on the results of any test of antimicrobial efficacy (8). Non-spore-forming organisms in log growth phase may differ dramatically in susceptibility from organisms in a static or dying culture. It is not advisable to use the same bacterial suspension for inoculum on several consecutive days because organism viability will decrease over time. Generally, standardized methods for testing antimicrobial effectiveness recommend that vegetative bacterial suspensions be prepared on the day of testing. It may be necessary to use a spectrophotometer to determine the approximate concentration in the suspension prior to testing.
Using an effective preparation method of spore suspensions, bacterial or fungal, is crucial to having a successful outcome and aids in the repeatability of the disinfectant qualification test results. If suboptimal procedures are used and the resulting spore suspension contains cellular debris or mycelial fragments, aggregation of spores may occur. It has been shown that spores that have aggregated are more difficult to disinfect than monodispersed spores (9). If spore aggregation occurs, it can be challenging for the biocide to penetrate and achieve adequate contact with the spores, leading to insufficient log reductions. It has also been concluded that hydrogen peroxide resistance in B. licheniformis can be increased when aggregation is present, as some spores may be protected by catalase produced by other spores in the aggregated mass (10). Additionally, if mycelial fragments are present in a fungal spore suspension, the fragments can shield the fungal spore from contact with the biocide. Prior to testing, it is advisable to confirm microscopically that the spore suspension contains at least 95% spores to provide an adequate challenge. Testing against the vegetative form of the spore may lead to overestimation of the effectiveness of the biocide against the fungal or bacterial spore isolate.
Gram-negative bacilli, such as Pseudomonas aeruginosa, Ralstonia pickettii, and Sphingomonas sp., are often isolated from wet environments. Subsequently, they often do not survive desiccation well, and in some conditions a greater than a 3 log10 reduction is observed due to the drying process (11). Given the potential impact of drying upon viability, it is critical to control the drying process for inoculated coupons as much as possible, particularly for organisms that are sensitive to desiccation. For instance, if the test coupon inocula are fully dried while the positive control coupon inocula are still wet, it is possible for a log reduction to be observed due to the differential drying process alone, leading to falsely high log reduction calculations by virtue of the reduction of viable cells in the overly dried test coupons. Meticulous evaluation of appropriate drying conditions can be challenging but is sometimes necessary in order to successfully complete a study. One factor that can mitigate the deleterious effects of drying is the presence of an organic soil load in the inoculum (12, 13). However, the presence of an organic soil load also provides an additional challenge to the disinfection process, and there should be a rationale behind the inclusion and amount of soil load included in a disinfectant efficacy study. One approach, which may be viewed by some, as a viable solution to the desiccation lethality issue, is testing against an inoculum without drying it. Testing against a liquid state inoculum is not recommended for multiple reasons. For instance, it is inadvisable to have standing water in a classified environment, so most contamination will be found on dry surfaces. Additionally, testing against a liquid inoculum is similar to a suspension test, in which 360 degrees of contact with the organisms in a liquid suspension is possible. Finally, when testing against a liquid inoculum, the concentration of the biocide is reduced proportionally to the ratio of biocide to liquid inoculum.
Because the lethality of some organisms after drying can be an issue, there is a chance that the starting inoculum over time will change and affect the overall results. Some organisms such as Pseudomonas spp. are very sensitive to drying. It is therefore necessary to determine how much inoculum will be lost due to drying for each organism on each surface type. Taking this into account will aid in preparing suspensions of an adequate concentration to show the necessary log reduction.
Surfaces
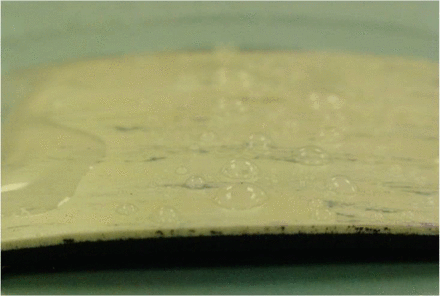
The surface tension of a biocide can lead to challenges in disinfectant qualification testing. Surface tension is a result of cohesive forces that exist between liquid molecules and is reflected in disinfectant qualifications by the surface area that a given volume of liquid covers when placed on a surface. A high-surface-tension liquid covers a smaller surface area than a low-surface-tension liquid. Beyond the inherent surface tension of a liquid, the interfacial tension should also be considered. Interfacial tension involves the adhesive forces of the biocide and the surface coupon. The differences in the interfacial tension between various biocides and surfaces can significantly affect the outcome of disinfectant qualification testing. If a high interfacial tension exists between a biocide and a surface coupon, it may have a tendency to bead up in small droplets. This can make attaining complete contact with the microorganism inoculum challenging and lead to failure to meet log reduction acceptance criteria due to the microorganism not being in direct contact with the biocide. Conversely, if a low interfacial tension exists between a biocide and surface coupon, the biocide can overrun the boundaries of the coupon and lead to difficulties in achieving the required wet contact time. This can also lead to failure to meet log reduction acceptance criteria due to inadequate contact of the biocide with the challenge microorganism. Knowing these effects prior to developing and executing a testing protocol can help to develop procedures that avoid these pitfalls. Log reduction failures that are due to test design, rather than to the biocidal activity of the test product, are a significant contributor to failing results.
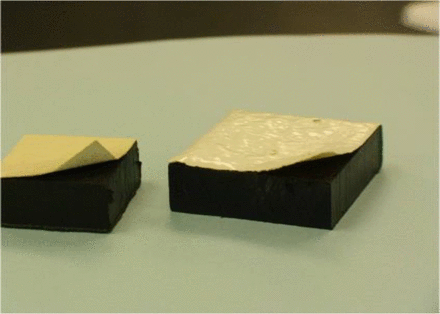
Epoxy paint peelingafter autoclaving.
Preparation of surfaces prior to testing can also affect the outcome of the study. It is important to clean the surfaces well with a neutral detergent and to rinse thoroughly. Sterilization is the next step. The use of an autoclave or a dry heat oven, although probably the easiest means of sterilization, may not be the best choice for all surface types because warping and peeling of the surface can lead to study failures. It is important to know the heat tolerance of the surface, which can usually be obtained directly from a manufacturer's technical literature. As an alternative to heat sterilization, the surface can be disinfected with a sporicide followed by a thorough sterile water rinse and a final application of 70% IPA. Application of hydrogen peroxide vapor may also be an option.
Most disinfectants and sporicides used in the life sciences industries are specifically recommended for use on hard, non-porous surfaces. Porous and damaged surfaces will pose a challenge, as the microorganisms can become lodged in crevices and cracks and be protected from contact with the biocide. However, if your cleanrooms contain damaged surfaces, regulatory authorities will expect that the same damaged surfaces be used in your study. The FDA has cited facilities that do not include damaged surfaces in their testing: “The materials that were tested in the Disinfectant Efficacy study were not representative of all the surfaces present in the Aseptic Processing Area. The stainless steel coupons tested did not represent these damaged surfaces” (14). Significant or repeated testing failures due to damaged cleanroom surfaces can often be the impetus needed to justify the expense of new surfaces.
Warped vinyl flooring/interfacial.
Neutralization
Neutralization validation is a critical component of any disinfectant qualification. The objective of neutralizer validation is to demonstrate that residual disinfectant is inactivated at the end of the contact time. If adequate neutralization is not achieved, the contact time being tested is not precise, as residual disinfectant activity will continue to occur beyond the prescribed contact time. This could result in overestimation of the log reduction achieved by the biocide and potentially lead to inadequate disinfection in the classified area. In addition to demonstrating the effectiveness of the neutralizer in the validation, it is essential to assess the toxicity of the neutralizer to microorganisms included in the disinfectant qualification. If the neutralizer is toxic to the microorganism, this lethality will be attributed to the biocidal activity of the test product, and an overestimation of the log reduction of the disinfectant or sporicide being tested will occur. Toxicity can be particularly problematic to sublethally injured cells, adding a secondary stress to the primary stress of the biocide being evaluated (15). Determining the toxicity of the neutralizer on a subset of the microorganisms being examined can also lead to flawed studies. It has been demonstrated that the toxicity of neutralization solutions have varied effects upon different organisms (16). Only examining the toxicity to the organisms that have been deemed representative or worst case can lead to specious log reduction values for the microorganisms, against which the neutralizer toxicity was not assessed. Neutralizer toxicity also can lead to low positive control recovery, making it impossible to demonstrate the required log reduction.
Recovery Method
Effective removal of remaining microorganisms from the surface is crucial. There are several methods that are used for recovering survivors, including rinsing, swabbing, contact plating, vortexing, and sonication. There are advantages and disadvantages to each method. Although recovery by contact plating or swabbing is relatively quick and easy, these methods are prone to variability. Many factors can contribute to poor recovery, such as the swab material (e.g., cotton, rayon, polyester, calcium alginate), variations in surfaces, and differences in analyst technique (e.g., pressure applied), which can contribute to unequal or incomplete removal of any surviving organisms (17). The method of choice should be easily reproducible and not prone to operator variation.
The selected method of recovery must demonstrate that the technique used to remove organisms from the coupons is effective and should be verified within the study with appropriate controls. Verifying the recovery method demonstrates that the microorganism inoculated onto the coupon surface can be recovered if present after exposure to the disinfectant. A sound recovery study will assure that organisms are not lost due to desiccation and/or inadequate recovery methods, which again can lead to overestimation of a biocide's effectiveness.
Conclusion
Considering the numerous factors that can lead to disinfectant effectiveness testing failures, it is critical that potential problems and test variables are considered and addressed before beginning the actual disinfectant effectiveness study. It is also often necessary to perform small experiments prior to testing to determine the appropriate neutralizer and recovery method for the study. Planning is key because a poorly executed study will likely provide no usable data and repeating a study requires another significant outlay of resources and time, in addition to carrying the regulatory risk of not having qualified biocides. Being thoroughly prepared up front will save time and resources later and lead to a study that can stand up to regulatory scrutiny.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2017