Background
The goal of Session 3.2 was to present alternative approaches to virus removal or inactivation that could be included as part of a production process for biologics. These approaches could include a new unit operation such as heat inactivation or a variant of solvent/detergent treatment, or the use of a unit operation that is already an integral part of an existing process such as flocculation or depth filtration, or even replacement of a process step with a more manufacturing-friendly but similar unit operation such as an anion exchange (AEX) membrane adsorber in place of an AEX column chromatography step.
Multiple Uses for Adsorptive Depth Filters in Early-Stage Programs (Anne Kantardjieff and Saravanamoorthy Rajendran; Alexion Pharmaceuticals)
The use of adsorptive depth filters was explored for multiple applications within early-stage programs. In the first application, the use of adsorptive depth filters to increase virus filter throughput was investigated. Insufficient throughput at the virus filter stage was identified for a fusion protein. The impact of load concentration, aggregate content, and particulate count on virus filter throughput was characterized, and a significant effect of all three parameters was identified. As a result, experiments were designed to minimize aggregate load on the virus filter. The use of an adsorptive depth filter at multiple locations in the process was tested, with the ultimate objective of maximizing viral filter throughput.
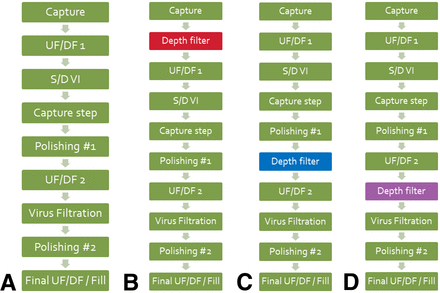
As shown in Figure 1, three different locations for the adsorptive depth filter were evaluated.
Evaluating the optimal location for an adsorptive depth filter. (A) Original process. (B) Schematic 1, with depth filter immediately following the capture step. (C) Schematic 2, with depth filter immediately following the first polishing step. (D) Schematic 3, with depth filter immediately prior to virus filter.
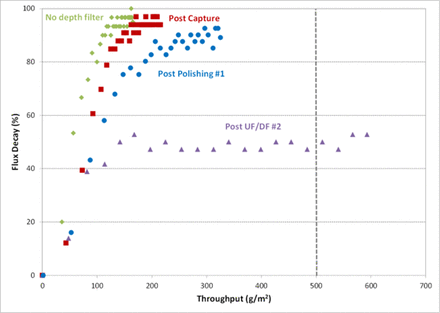
The impact of depth filter placement on virus filter flux decay is shown in Figure 2. No significant impact was observed when placing the depth filter either following the capture step or first polishing step. However, a substantial increase in virus filter throughput was observed when the adsorptive depth filter was placed immediately prior to the virus filter.
Impact of depth filter placement on virus filter flux decay.
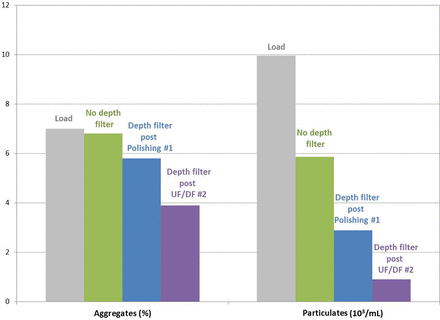
The level of aggregates and particulates after each depth filtration step was characterized using size exclusion chromatography and micro-flow imaging (MFI), respectively. As can be seen in Figure 3, a significant removal of both aggregates and particulates was observed when the adsorptive depth filter was placed immediately prior to the virus filter. Based on these results, an adsorptive depth filter was added to the process, directly before the virus filter. A risk assessment was performed to evaluate the impact of adding this filter on extractables and leachables, and it was concluded that the final polishing step provided sufficient clearance capability.
Impact of depth filter placement on the level of aggregates and particulates.
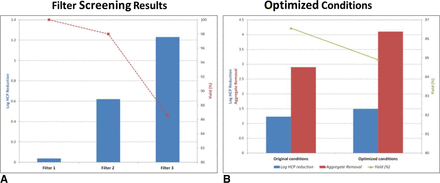
In a different application, the use of an adsorptive depth filter was examined for host cell protein (HCP) removal. Following a process change at the production bioreactor stage, a higher level of HCPs was observed in the bulk drug substance. Three adsorptive depth filters were evaluated for their impact on HCP reduction (Figure 4). A depth filter with anionic properties was selected for future evaluation (Filter 2). Filter loading and load pH were optimized to maximize impurity removal while maintaining greater than 85% yield.
Depth filter screening for host cell impurity clearance. (A) Filter screening results for log10 HCP reduction and process yield. (B) Log10 HCP reduction, aggregate removal, and process yield for Filter 2 under original and optimized conditions.
Xenotropic murine leukemia virus (X-MuLV) and minute mouse virus (MMV) clearance across the depth filter was subsequently evaluated at the final operating conditions of ≤150 L/m2 loading, pH 7.0, and conductivity of 15.6 ms/cm. As shown in Table I, minimal clearance was observed for both MMV and X-MuLV. It should be noted that the operation conditions were optimized for impurity removal and process yield, not viral clearance. Of note, it was observed that process yields were significantly reduced at lower pH.
Viral Clearance Results Using the Adsorptive Depth Filter 2
In conclusion, it was shown that adsorptive depth filters can be utilized in support of an overall viral clearance strategy. Notably, they can provide significant benefit for early-stage programs in which speed is critical. While viral clearance was poor under the conditions we evaluated, there is a potential to optimize operating conditions to maximize viral clearance. However, the applicability of this approach remains to be seen, as raw material variability could potentially lead to variability in viral clearance performance.
Development of Alternative Virus Clearance Unit Operations to Meet the Evolving Business Need (Dayue Chen; Lilly Research Laboratories, Bioprocess Research and Development)
Viral clearance provides assurance that biologics manufactured using a mammalian cell culture process are free of viral contaminants. A typical downstream purification process contains at least three unit operations capable of removing or inactivating viruses in order to ensure a robust and reliable virus clearance capacity. Unit operations involved in virus removal/inactivation can be divided into two classes based on their primary roles in purification processes: dedicated or non-dedicated. The only role of dedicated unit operations, such as viral filtration or low-pH inactivation, is to remove or inactivate viruses. The primary function of non-dedicated unit operations, such as Protein A chromatography or AEX chromatography, is to enrich products and/or remove impurities, but they can also contribute to the overall viral clearance capacity of the purification process. As pipeline molecules become more varied and complex in nature, and with growing environmental concerns over the use of certain reagents in the production process, such as Triton X-100, which can be used to inactivate certain virus types, it has become increasingly necessary to develop additional, alternative unit operations that are capable of clearing viruses. To this end, we have evaluated four different methods in our laboratory to determine whether they can be implemented in downstream processes for virus clearance, including (i) heat inactivation, (ii) depth filtration, (iii) Polysorbate 80 (PS80) + tri-n-butyl phosphate (TnBP) inactivation, and (iv) flocculation.
X-MuLV was spiked directly into cell-free bioreactor harvests and then incubated at different temperatures. Time point samples were taken to determine the inactivation kinetics. X-MuLV infectivity was quantified on PG-4 cells by TCID50 assay as previously described (1). As shown in Table II, X-MuLV can be effectively inactivated by heat treatment at moderate temperatures. The extent of inactivation is directly related to temperature and duration.
X-MuLV Inactivation by Heat Treatment
Both X-MuLV and porcine parvovirus (PPV) were used as model viruses to evaluate whether depth filtration can provide reliable virus clearance. Under a variety of pH and conductivity conditions and with two different filters, the level of retrovirus clearance achieved was consistently around 3 log10, while clearance of parvovirus was dependent on the filter type and operating conditions. The results from these studies have already been published (2).
Flocculation was also evaluated for virus removal using X-MuLV and PPV as model viruses. Smart polymers used in the flocculation experiments along with detailed procedures have been described previously (3). X-MuLV and PPV were titrated on PG-4 cells and PK-13 cells, respectively, by TCID50 method as previously described (1). As shown in Table III, a modest clearance of retrovirus and parvovirus was achieved (2–2.5 log10) when flocculation was performed using a “smart” polymer. No virus clearance was observed when flocculation was performed without the “smart” polymer.
Virus Clearance Achieved by Flocculation
Solvent and detergent (S/D) is a proven unit operation for virus inactivation commonly used in the plasma industry (4). PS80 in combination with TnBP was evaluated for retrovirus inactivation in place of Triton X-100, which is the standard detergent used in this unit operation. As expected, X-MuLV was rapidly inactivated to below the limit of detection even at the lowest concentration evaluated (Table IV).
X-MuLV Inactivation by PS80 and TnBP
In summary, four different methods for virus clearance were evaluated. Heat and S/D treatments were capable of providing reliable and robust retrovirus clearance, representing potential alternative unit operations for use in production to ensure adequate virus clearance. On the other hand, depth filtration and flocculation are much less effective in comparison.
Maximizing Productivity with AEX Membrane Adsorbers—Impact on Robustness and Edge of Failure? (William Rayfield, David Roush, *Adrian Gospodarek, *Mark Brower, Collette Cutler, and Nihal Tugcu; Process Development & Engineering and *Biologics Technology & Expression, Bioprocess R&D, Merck Research Laboratories)
Flow-through AEX chromatography is often used as a polishing step during purification of monoclonal antibodies (MAbs). AEX chromatography removes residual impurities (HCP, DNA, and Protein A ligand) and viruses, which is an important factor that could affect the safety of a biologic and is critical to support assessment by regulatory agencies (5). Traditional AEX column chromatography utilizes positively charged ligands that bind the negatively charged impurities and viruses while the positively charged MAb is not retained and is recovered in the flow-through. AEX column chromatography can often achieve effective virus reduction: >4 log10 reduction value (LRV) (6). Membrane adsorbers are a promising alternative to columns for MAb purification that can increase productivity while maintaining impurity clearance. The convective flow properties of membranes allow for higher operating flow rates and effective MAb loading; however, a more limited data set exists on viral clearance for this technology (7, 8). This section of the paper summarizes a case study evaluating the partitioning of a four virus panel—MMV, X-MuLV, Reovirus Type 3 (Reo3), pseudorabies virus (PrV)—for a MAb process using several marketed AEX membrane adsorbers across a range of operating conditions (impurity load, flow rate, feed pH, and conductivity) to show process robustness in comparison with traditional AEX column chromatography.
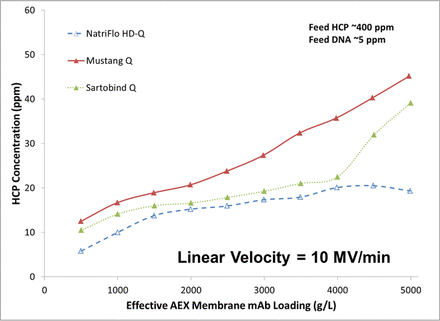
Three AEX membrane adsorbers (Natrix NatriFlo HD-Q, Pall Mustang Q, and Sartorius Sartobind Q) were evaluated for use in the late-stage purification process for MAbs A and B (Table V). While both MAbs had utilized AEX resin for the early-stage process, AEX membranes have potential time and cost advantages, are disposable, and can provide effective HCP reduction at high loading. However, AEX membranes are applicable for specific applications (HCP/DNA removal), and have limited resolution for product-related variants (high molecular weight species) under the conditions tested and have been shown in the literature to have lower LRV for some model viruses (such as MMV) as compared to traditional AEX resin (9). From an internal cost analysis using Biosolve software, a target MAb loading of ≥2000 g/L was necessary for AEX membrane cost to be comparable to AEX resin (facility operations, buffer volumes, cycle time, etc.) (10). Unspiked MAb runs (Figure 5) using HCP breakthrough as a surrogate for virus breakthrough suggested that loading in excess of 2000 g/L was possible.
Comparison of MAb and AEX Processing Parameters
HCP reduction during unspiked MAb A AEX adsorber runs as a function of loading at recommended vendor linear velocity.
Despite previously published research, a knowledge gap in the literature existed for viral clearance data on AEX membrane adsorber technology (AEX membranes are still considered an emerging technology); therefore, a study was designed to evaluate several key operating parameters at the edge of failure: MAb/impurity loading, flow rate, conductivity, and stationary phase. The study was split into two stages: (1) a spiked buffer (placebo) run on all three AEX membranes to define the upper theoretical limit of LRV (i.e., virus-ligand binding in the absence of MAb), and (2) a robustness study (Table VI) evaluating LRV performance as a function of loading under control and challenge conditions using a four-virus panel: X-MuLV, MMV, PrV and Reo3. All AEX runs were fractionated and collected, with membrane runs sampled at 2000, 4000, and 6000 g/L with a level of quantitation (LOQ) of ∼2 LRV. Representative pools were created based on the full collection volume and a large-volume sample was taken (LOQ ∼1.2 LRV).
Comparison of MAb and AEX Processing Parameters
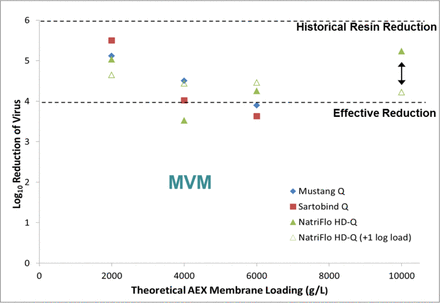
The spiked buffer runs suggested high LRVs in the absence of MAbs, with reduction of X-MuLV, PrV, and Reo3 viruses to below LOQ for all three AEX membranes throughout the loading (data not shown). Some background breakthrough was observed for MMV (Figure 6), and higher viral loading reduced the LRV (arrows in Figure 6). Based on the buffer run data, the same loading was used for the MAb runs.
LRV as a function of theoretical AEX membrane loading during spiked buffer runs (10 MV/min).
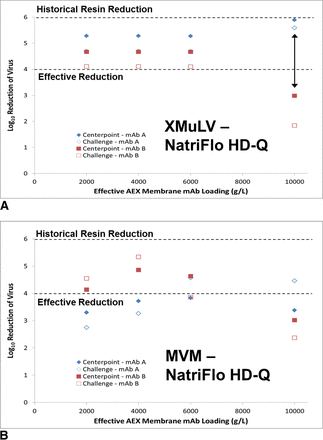
For stage 2 of the study, virus removal (LRV) varies with MAb and virus type. For X-MuLV (Figure 7A), PrV, and Reo3 (data not shown), virus levels in the NatriFlo HD-Q membrane filtrate pool were below the LOQ at loadings of up to 6000 g/L for MAb A, and up to 4000 g/L for MAb B. Virus partitioning was observed in all filtrate fractions for MMV (Figure 7B), with loss of effective reduction (defined as ≥4 LRV) at a load of 2000 g/L for MAb A and 4000 g/L for MAb B (MAb B statement based on data not shown). While the data from membrane fractions suggests that additional virus is unbound during MAb B loading, it also suggests that some additional preferential binding of virus is occurring with increasing MAb A loading (Figure 7B).
LRV as a function of effective AEX membrane loading during spiked MAb runs on Natrix NatriFlo HD-Q absorber for (A) X-MuLV and (B) MMV.
The results from this case study suggest an effective LRV (≥4 log10) for X-MuLV, PrV, and Reo3 viruses can be achieved at a load challenge up to 6000 g/L on the three AEX membrane adsorbers tested. The results also confirm previously published findings that indicate reduced MMV clearance as compared to AEX resins (7). The use of AEX membranes is an option for well-behaved systems in which high molecular weight species reduction and high MAb loading are unnecessary. Additional work is needed to evaluate the MMV-MAb-ligand interaction to better understand the cause of reduced MMV LRV on AEX membrane adsorbers.
Initial Characterization of Viral Clearance Using Adsorptive Depth Filters (John Schreffler and Tom Klimeka; aEisai, Inc.)
The use of adsorptive depth filters (ADFs) to remove precipitants and impurities following Protein A capture is becoming more common in biopharmaceutical manufacturing. In most antibody purification processes, low-pH virus inactivation is performed after capture with a Protein A resin. Significant precipitation of process-related impurities can occur during the low-pH adjustment and subsequent conditioning step, so depth filtration is often required before performing subsequent polishing chromatography steps. Previous experiments in our laboratory have demonstrated that ADFs can remove a large proportion of impurities (HCPs, host cell DNA, and residual Protein A ligand) before processing over an AEX matrix, thus making the AEX step redundant except for viral clearance. Viral clearance capabilities of ADFs have been previously demonstrated, but the mechanism or effectiveness has not been thoroughly investigated. An initial study was performed that assessed the viral clearance capabilities of Millipore X0HC depth filters using a series of experiments designed to characterize X-MuLV and MMV clearance levels. X-MuLV and MMV clearance was measured across three molecules with load pH ranging from 6.5 to 7.2, low load conductivity (≤5 ms/cm), and loading levels up to 100 L/m2, under constant flow conditions. Clearance of MMV varied from 1.1 to 6.9 log10 reduction factors (LRF) across the three molecules, with increased MMV removal observed with increasing load pH, indicating that MMV clearance by the X0HC depth filter is mainly achieved via charged based interactions between the virus and filter. Alternatively, complete removal of X-MuLV was achieved for all three molecules. Clearance of X-MuLV appeared to be independent of load pH and load ratio, indicating that X-MuLV clearance may involve a mechanism other than electrostatic interactions. Viral clearance results are summarized in Table VII.
Testing with Mab-A Compared Multiple Filter Lots in Order To Examine Lot-to-Lot Variability
To date, a viral clearance claim has not been made for the Millipore X0HC depth filters. Results from this initial study showed robust clearance of X-MuLV under varying load conditions, but clearance of MMV was more variable, with initial data providing evidence that clearance is largely due to charge-based interactions between the virus and filter. Future work will include additional studies to better under the mechanism for both impurity removal and viral clearance, and evaluation of a new version of synthetic X0HC depth filter.
Points To Consider for Virus Clearance When Switching from Packed Bed Chromatography to a Membrane Adsorber (Sumiko Hasegawa, Keiji Iwamoto, Takuya Muramoto; Hikari Bio-manufacturing Technology Laboratories, CMC Center, Takeda Pharmaceutical Company Limited)
Biotechnology products derived from a cell culture production process have a potential risk of adventitious virus contamination, which leads to serious clinical issues. To minimize the risk to patient safety, a downstream purification process needs to be developed with a capacity to remove contaminants such as viruses as well as impurities such as HCPs, host cell DNA, and product aggregates. AEX chromatography is a unit operation that is often utilized in downstream purification processes because of its ability to remove the abovementioned contaminants and impurities. AEX chromatography can be accomplished by using either a packed bed resin, which is considered the more standard approach, or, as an alternative, an AEX membrane adsorber. Membrane absorbers can be easily scaled up and, as compared to a packed bed resin, can be processed at much higher flow rates due to their structural characteristics. In addition, salt-tolerant AEX membrane adsorbers, such as the Sartobind STIC, have recently become available. These salt-tolerant AEX membranes appear to have a broader operating range as compared to the more traditional AEX resins and membranes, which have a salt-sensitive, quaternary amine ligand.
A reduction in viral clearance was observed as a consequence of switching to the Sartobind STIC from a packed bed AEX resin in a MAb production process. Although load pH and conductivity were ruled out as having any impact, several other factors—including the buffer component (different from the AEX packed bed chromatography step), virus spike ratio, and virus preparation—may have had a negative impact on the capacity of the AEX membrane step to remove virus. Additional follow-up studies indicated no impact to the AEX membrane step performance by the changed buffer component or the virus spike ratio. In regards to the quality of the virus spike itself, impurities in the virus stock such as serum proteins and media components may influence the outcome of the virus clearance study (11). Additional studies were performed to investigate the effect of virus stock preparation on viral clearance. In an effort to remove impurities, the virus stock was further purified using an ultracentrifugation (U/C) method. This additional level of purification of the virus stock led to a significantly improved level of virus clearance over the salt-tolerant AEX membrane (>4.4 log10). This result suggests that impurities in the original (no U/C) virus stock may interfere with virus binding to the ligand on the AEX membrane and, as a result, the virus remains in the flow-through fraction with the product, leading to a reduced level of viral clearance. The use of an AEX membrane may also exaggerate the possible impact that impurities have on viral clearance as compared to an AEX packed bed resin. As the load volume and mass challenge for an AEX membrane step is usually much greater per unit volume than for an AEX packed bed resin step, more impurities will also be loaded onto the membrane absorber per unit volume than with the packed bed column. As a result, the less purified virus stock (no U/C) likely has an even larger impact on viral clearance over the AEX membrane as compared to an AEX packed bed resin. Based on these findings, the quality of the virus stock used for virus clearance studies should be considered, especially when large volume/mass challenges will be applied to an AEX membrane step.
Summary
The virus removal/inactivation potential of several alternative processing steps was discussed.
In the first presentation, placement of an adsorptive depth filter immediately prior to a virus filtration step led to a substantial increase in virus filter throughput as compared to no significant impact when the depth filter was placed further upstream in the process. The benefit of the depth filter immediately preceding the virus filtration step is believed to be due to removal of product aggregates and particulates from the load material to the virus filter. Depth filters from several different vendors were also evaluated for their ability to remove HCP and model viruses, with some level of success in removing HCP but less effective removal of viruses. In addition, there is general concern regarding depth filter performance due to raw material variability and its potential impact on viral clearance.
In the second presentation, four alternative unit operations including heat inactivation, depth filtration, polysorbate 80 + TnBP, and flocculation were evaluated to determine their viral clearance capability. Using the heat inactivation approach, a temperature of 50 °C was found to be very effective against a model retrovirus, while lower temperatures (40–41 °C) were less effective. Depth filtration could provide a high level of viral clearance depending on the type of model virus tested (retrovirus or parvovirus), the type of depth filter, and operating conditions. Under a variety of operating conditions and using two different filters, a 3 log10 retrovirus clearance was consistently achieved, while clearance of parvovirus was dependent on the filter type and operating conditions. These results are not included in this meeting summary but have already been published (2). The polysorbate 80 and TnBP solvent/detergent (S/D) treatment was equally effective as the Triton X-100 and TnBP S/D treatment in rapidly inactivating a model retrovirus. And, lastly, flocculation alone showed no capability to remove virus, but when combined with a “smart” polymer, a modest level of retrovirus and parvovirus clearance was achieved (2–2.5 log10). In summary, both heat and S/D treatment appear to be reliable and robust options for retrovirus clearance, while depth filtration and flocculation are much less effective.
In the third presentation, a case study was presented that evaluated clearance of four model viruses (MMV, X-MuLV, Reo3, PrV) over several AEX membrane adsorbers operated under a wide range of operating conditions. Two MAbs were tested with three different AEX membranes including Natrix NatriFlo HD-Q, Pall Mustang Q, and Sartorius Sartobind Q. Results from this study suggested a high level of X-MuLV, PrV, and Reo3 clearance can be achieved by all three AEX membranes up to a load challenge of 6000 g/L. These results appeared to be similar to the level of clearance achieved by AEX chromatography resins. Conversely, MMV clearance over the AEX membranes was reduced as compared to the other model viruses and reduced in comparison to clearance obtained with AEX resins.
Using two model viruses, X-MuLV and MMV, the fourth presentation focused on the characterization of viral clearance over a Millipore X0HC depth filter. Under constant flow conditions, viral clearance was evaluated using three different antibodies over a given pH range (6.5–7.2), low load conductivity (≤5 ms/cm), and a load challenge up to 100 L/m2. The investigators found that clearance of MMV varied, with increased clearance observed with increasing pH. Conversely, pH appeared to have no impact on the level of X-MuLV clearance achieved—it was high across the pH range tested. Results from this study showed robust clearance of X-MuLV over a range of conditions, while clearance of MMV was more variable, with the data suggesting that clearance is due more to charge-based interactions between the virus and filter.
The final presentation of this session focused on a switch from a packed bed AEX chromatography resin to a salt-tolerant AEX membrane adsorber, Sartobind STIC. The initial results from this evaluation indicated a lower reduction of viruses over the AEX membrane as compared to the AEX resin. Upon further investigation, it appeared that purity of the virus stock used for the study was largely responsible for the lower level of virus clearance achieved by the AEX membrane. Use of a virus stock that had been prepared by U/C led to a significant increase in viral clearance. Because the ratio of load challenge to AEX membrane volume is much greater as compared to an AEX column volume, the level of impurities in the virus stock may have a greater impact on the AEX membrane performance as compared to the performance of a packed bed AEX resin. Consequently, the quality of the virus stock needs to be considered when evaluating viral clearance capability of an AEX membrane step.
- © PDA, Inc. 2016