Background
Chromatographic purification steps can significantly contribute to the viral clearance profile of biotechnological therapeutic proteins. International Conference on Harmonisation (ICH) guideline ICH Q5A specifies consideration of the performance of recycled resin for viral clearance (1) and, in practice, robustness of virus reduction with respect to resin age has routinely been assured by performing viral clearance studies using new as well as used or recycled resins. If fully executed, these studies for chromatographic steps can examine the impact of not only new versus used resin, but also the effectiveness of column cleaning, the distribution of virus across the step, and examination of virus carryover. In total, these studies can add up to many column runs and, in the case of used resin, require a significant amount of time and material to generate data for various model viruses.
Review of Viral Clearance Strategies for Cycled Chromatography Resins (Glen Bolton; Amgen)
Some contract viral testing labs have taken the stance that “The viral reduction capacity of [chromatography] resin at the limit of its reuse must … be shown to be equivalent or greater than with new resin” and that “data must be generated before a product is licensed” (2). However, this level of specificity on how to achieve the goal of assuring chromatography robustness with respect to resin age is not actually written into existing guidance. While ICH Q5A describes “Some estimate of the stability of the viral clearance after several uses may provide support for repeated use of such columns” (1), it doesn't directly specify that viral clearance data using resins at the limits of reuse (i.e., aged resins) are the only way to reach this goal.
It is true that an estimate of the viral clearance capacity of reused media as described by ICH Q5A can be obtained by performing small-scale virus removal studies using both new and reused column media. However, a second, simpler approach to obtaining this estimate has been proposed by performing virus removal validation studies on new media only and then monitoring chromatography performance attributes such as product step yield, resolution, or eluate impurity content during production that might decay prior to virus clearance. There is a significant body of literature that has accumulated in the past 20 years since ICH Q5A was written that consistently indicates the latter approach is sufficient for estimating the viral clearance capacity of used resin of certain types (e.g., protein A, anion exchange) without increasing process risk.
Brorson et al. (3) demonstrated no effect of 150 or 460 cycles on the clearance of endogenous retrovirus viruses in a monoclonal antibody (mAb) process using protein A resin. Lifetime was observed to be limited by a decline in step yield while clearance of endogenous retrovirus was unchanged. Lute et al. (4) demonstrated no effect of up to 300 cycles on the clearance of endogenous retrovirus viruses, xenotropic murine leukemia virus (X-MuLV), minute virus of mice (MVM), and Simian virus 40 (SV40) in a mAb process using four different protein A resins. Media lifetime was observed to be limited by either a decline in step yield or fouling. In these cases, clearance of endogenous retrovirus either increased or was unchanged. Zhang et al. (5) demonstrated no effect of up to 250 cycles on the clearance of endogenous retrovirus viruses in a mAb process using protein A resin.
Kelley et al. (6) described no apparent effects of cycling a peptide ligand affinity resin on the clearance of four viruses in a recombinant factor VIII (rFVIII) process. Morrica et al. (7) demonstrated no effect of 16 cycles on the clearance of canine parvovirus (CPV) and bovine viral diarrhea virus (BVDV) in an anti-thrombin III process using a heparin sepharose resin. Andersson et al. (8) demonstrated no effect of 190 cycles on diethylaminoethanol (DEAE) resin or of 440 cycles on CM Sepharose FF on the clearance of bovine herpes virus (BHV), BVDV, encephalomyocarditis virus (EMCV), and human immunodeficiency virus (HIV), and on CPV and BVDV in a plasma albumin and IgG purification process. Xie et al. (9) demonstrated no effect of 476 cycles on the clearance of dengue virus from a plasma albumin and IgG purification process using a cation exchange (CEX) resin.
Norling et al. (10) demonstrated no effect of 200 cycles on the clearance of MVM and SV40 viruses in a mAb process using Q-Sepharose fast flow, Q-Ceramic Hyper D, or Toyopearl Super Q-650 M resins. However, when the Q-Sepharose resin was cleaned with an HCl solution that degraded the resin or not cleaned at all, viral clearance gradually declined. For these cases, changes in routinely measured attributes including loss of chromatographic resolution as measured by HETP (height equivalent to a theoretical plate), DNA residuals in the column effluent, and back pressure would contraindicate further column cycling in advance of an impact on viral clearance.
Kelley et al. (11) demonstrated no consistent reduction in viral clearance for used resin by surveying a broad set of company data. The data comprised 90 different runs performed with 10 different chromatographic resins, 3 products, and 6 viruses. They proposed that viral clearance data with cycled resins would not be warranted if certain criteria were met:
Lab-scale cycling data demonstrated consistent performance over the column lifetime.
Routine monitoring of column performance was carried out in current good manufacturing process (cGMP) manufacturing.
The product was derived from a well-characterized host.
The process lacked human- or animal-derived raw materials and contained at least one robust viral inactivation step.
Based on these studies covering a broad range of chromatography resins, therapeutic products, and virus types it appears that after a technical assessment, a conclusion can be drawn that monitoring of chromatography performance during routine production may be adequate to ensure viral clearance capacity of well-understood resins like protein A after cycling. This has been adapted by the Food and Drug Administration (FDA) Office of Biotechnology Products in their internal processes. In cases where a technical assessment indicates increased risk (11), a limited set of studies, perhaps with one or two viruses and with a partially cycled resin, may be justified based on the extensive literature data indicating the robustness of viral clearance of cycled resins.
Optimization of Viral Clearance Strategies (Martina Kopp; Amgen)
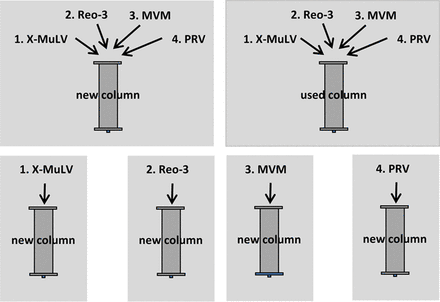
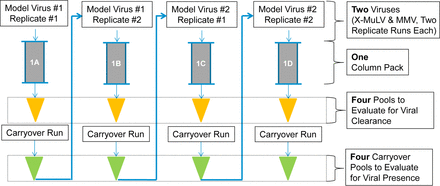
The overall industry need for viral clearance evaluation is predicted to more than double in the next five years. In this context, Amgen argues that new approaches will be needed to allow for operational efficiencies that can increase throughput and speed to further understanding while maintaining viral safety. Such opportunities for new approaches are the reuse of resins used in chromatography steps for subsequent spiking challenges with multiple model viruses as well as the elimination of the used resin testing and carryover studies. In the study presented at the Cambridge meeting, a comparative analysis was performed to test virus clearance and distribution on Protein A and CEX chromatography steps by comparing new resin with single-virus spike to multivirus spike and comparing new resin to used resin with multivirus spikes (Figure 1). The model viruses tested were MVM, reovirus type 3 (Reo-3), pseudorabies virus (PRV), and X-MuLV. Virus distribution was analyzed by quantitative polymerase chain reaction (qPCR) for all viruses. The log reduction values (LRVs) of enveloped viruses were assessed by utilizing qPCR for the Protein A resin, and infectivity testing was utilized to assess the LRV of non-enveloped viruses on Protein A resin and for all viruses on the CEX resin. Cleaning and regeneration efficiency were assessed on Protein A and CEX chromatography steps by infectivity testing of the carryover fraction.
Multivirus experimental plan.
No significant differences were observed in the LRVs for all viruses between new and used resin when spiked with multiple viruses as well as between new resin spiked with a single virus and new resin spiked with multiple viruses as shown in Table I. Distribution studies on the Protein A and CEX chromatography steps further demonstrated that virus distribution for X-MuLV, Reo-3, MVM, and PRV was comparable between multivirus spikes and single-virus spikes, and no differences could be observed between new and used resin. All four viruses could be shown to both bind to the CEX column and be released to various degrees into the elution pool. Only Reo-3 virus bound nonspecifically to or was entrapped within the Protein A column to a significant degree, whereas X-MuLV, Reo-3, and PRV were mostly detected in the flow-through fractions.
LRV for Protein A and CEX Columns
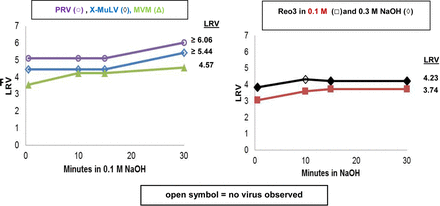
Amgen also presented data on the effectiveness of sodium hydroxide (NaOH) cleaning solutions that are used to clean columns and prevent lot-to-lot carryover of protein and virus. The data obtained demonstrated that no infectious virus (X-MuLV, MVM, Reo-3, and PRV) was detected in the carryover fractions. Additional in-solution inactivation studies using 0.3M and 0.1M NaOH showed complete inactivation of the enveloped viruses PRV and X-MuLV and more than 4 logs inactivation of the non-enveloped viruses MVM and Reo-3 (Figure 2). Taken together, the combined package of on-column and in-solution cleaning demonstrates that effective inactivation occurs for each virus.
In-solution inactivation of viruses.
In summary, no differences were observed between new and used resin and this is consistent with historical and previously published data. No carryover virus was observed for any virus tested using platform cleaning and storage solutions, and the in-solution data support effective inactivation.
Viral Clearance Studies with New and Cycled Resins at Biogen (Brad Stanley; Biogen)
Historical Biogen viral clearance data for cycled chromatography resins was summarized for the Cambridge meeting presentation. The data demonstrate that column cycling does not negatively affect the viral clearance capability of the resin. Our data include 95 comparisons of new versus cycled chromatography resins, with results from nine different biopharmaceuticals.
For this analysis, a significant difference in viral clearance for new and cycled resin was defined as greater than a 1 log. The results of individual comparative studies were broken down into three categories: (1) new/used resin results within 1 log; (2) results with no detectable difference in viral clearance capability of the step because the output effluent was nondetect (i.e., the study resulted in a “greater than” value, indicating no detectable virus); and (3) new/used resin results greater than 1 log different. Overall, 60/95 results demonstrated less than 1 log difference (category 1), 29/95 results demonstrated no detectable difference (category 2), and the remaining 6/95 demonstrated >1 log difference (category 3, the type that warrants additional consideration). Of the six with >1 log difference, only one was in the direction of a decline in clearance. A single case of LRV decline out of 95 studies could be viewed as an outlier and is certainly not indicative of a general trend. For both the meeting presentation and analysis, the historical viral clearance data were broken down by chromatography modes, including affinity, ion exchange (IEX), and hydrophobic interaction (HIC).
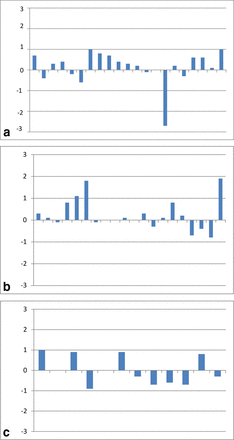
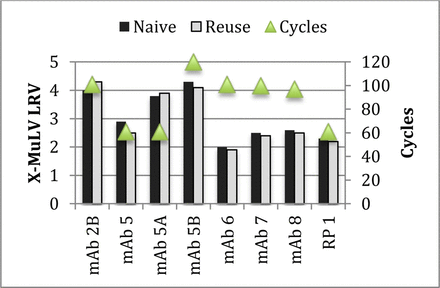
The Protein A affinity chromatography comparative analysis breakdown was as follows: 20/23 comparisons were within 1 log; 2/23 had no detectable virus in either sample; and 1/23 had greater than a 1 log difference (Figure 3a). Protein A affinity chromatography contained the only significant decline in viral clearance observed in all of the Biogen resin cycling data. This result indicated a 2.7 LRV decrease in the X-MuLV viral clearance capability of the step. While the overall viral clearance capability of the process was not affected significantly enough to warrant rerunning the study, the result stands out in comparison to the other Protein A steps analyzed, as well as published literature. The calculated LRV with new resin was the highest value observed across all of the Protein A data. The new and used resin LRVs were 5.2 and 2.5, respectively, while the averages were 3.0 and 3.2, respectively. The three other viruses tested on the same new and cycled resin all demonstrated results within 1 log. It is most likely that an unspecified anomalous result was obtained for the new resin but was difficult to explain or follow-up on.
Difference in LRV for new versus cycled resins. The LRV determined from the viral clearance study with new resin is subtracted from the LRV determined with cycled resin. Positive values indicate improved LRV with cycled resin while negative values indicate lower LRV with cycled resin. (a) Protein A resin, (b) IEX resin, (c) HIC resin.
The IEX chromatography analysis breakdown was as follows: 20/43 comparisons were within 1 log; 23/43 had no detectable virus in either sample; and 3/43 had greater than a 1 log difference (Figure 3b). All three studies that indicated greater than 1 log difference demonstrated an improved LRV with the cycled resin.
The HIC analysis breakdown was as follows: 12/16 comparisons were within 1 log; 4/16 had no detectable virus in either sample; and there were no observations of greater than a 1 log difference (Figure 3c).
The remaining 13 comparisons that do not fall into the Protein A, IEX, or HIC buckets above demonstrated the following: 9/13 comparisons were within 1 log; 2/13 had no detectable virus in either sample; and 2/13 had greater than a 1 log difference. The two studies that indicated greater than 1 log difference demonstrated an improved LRV with the cycled resin.
In summary, a review of all available viral clearance data from new versus cycled chromatography resins at Biogen demonstrates that cycling does not negatively affect the viral clearance capability of the chromatography step. These data are consistent with previously published studies (3, 4, 10, 11) and demonstrate limited value for end-of-resin-lifetime viral clearance studies.
Consistent Virus Clearance in Reuse Resins (L. Norling and Q. Chen; Genentech)
ICH Q5A states that “Over time and after repeated use, the ability of chromatography columns and other devices used in the purification scheme to clear viruses may vary. Some estimate of the stability of the viral clearance after several uses may provide support for repeated use of such columns”. Roche-Genentech historical virus clearance data for cycled chromatography resins were evaluated to assess virus clearance capacity of several resins after extended reuse in comparison with naïve resins. A summary of the assessment was presented at the Cambridge meeting.
At Roche-Genentech, chromatography column reuse studies are performed at reduced scale. Columns are run using representative feedstock and the same buffers, operation sequence, and parameters used in manufacturing. Chromatography column performance is assessed by taking samples at regular intervals throughout the study and analyzing them for yield and impurities. After a study is completed, the end-of-use resin is evaluated for virus clearance.
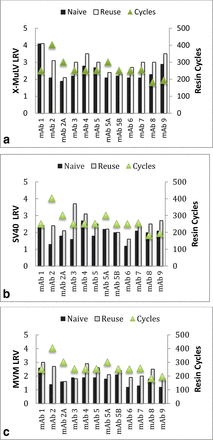
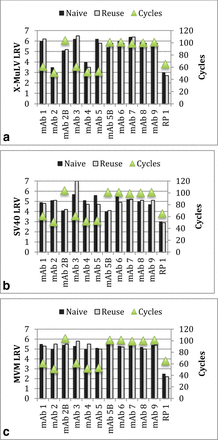
Affinity chromatography resin: Figures 4a-c summarize the virus removal by naïve and used protein A affinity resin. Protein A processes using nine mAbs and two types of protein A resins were studied. The data demonstrate that virus removal by protein A affinity chromatography is not affected by used resin up to 403 cycles for three model viruses. The LRVs obtained for the reuse resin were higher or comparable to those for the naïve resin.
Virus removal by Protein A resins. (a) X-MuLV, (b) SV40, (c) MVM.
Anion exchange (AEX) chromatography resin: Virus clearance studies for new and used (end-of-life) AEX resin run in flow-through or bind-and-elute modes are shown in Figures 5a-c. With nine mAbs, one recombinant protein (RP), and three AEX resins, used resin up to 104 cycles did not affect removal capacity of three model viruses.
Virus removal by AEX resins. a: X-MuLV, b: SV40, c: MVM.
CEX chromatography resin: X-MuLV clearance for new and used (end-of-life) CEX resin is shown in Figure 6. Used resin up to 120 cycles with five mAbs, one RP and four CEX resins did not affect X-MuLV LRV.
Virus X-MuLV by CEX exchange resins.
Conclusion: Roche-Genentech historical data show that resin reuse has no impact on virus clearance capability. In addition, product quality (impurities) is not affected by resin reuse small-scale studies (data not shown). The resin reuse limits are confirmed at full-scale for acceptable impurity clearance. Our data support the contention that there is no need to perform virus clearance studies with cycled Protein A, AEX, or CEX chromatography resins.
An Alternative Approach to Evaluation of Viral Clearance on New versus End-of-Life AEX Chromatography Resin (C. Gallo, A. Sacramo, and S. Sun; Pfizer)
The current approach taken by most companies to comply with ICH Q5A is to perform viral clearance studies on end-of-life chromatography resin to confirm no significant change in viral clearance capacity has occurred over the proposed resin lifetime. Studies evaluating virus carryover between cycles of resin use are also often performed to comply with language in ICH Q5A but may be waived if sufficient information regarding virus inactivation is available for the given resin cleaning and sanitization regime. Viral clearance studies using end-of-life resin (maximally cycled resin) typically require significant time, resources, and materials to generate the used resin needed to support these studies. As a result, sufficient maximally cycled resin and load materials are needed to support replicate column runs for each model virus, which usually involves three or four different model viruses. If a new, unused resin is also included in these studies as a control, then an even greater amount of materials are required. Pfizer developed an alternative approach to evaluate viral clearance on end-of-life resin that is more efficient and uses fewer resources and materials. Several aspects of the study design have been modified from the conventional approach including:
the use of only two model viruses as opposed to three or four model viruses,
the use of only one packed column for maximally cycled resin and one packed column for unused (new) resin.
As three or four model viruses are typically used in the original viral clearance validation study (using new resin), we have chosen to use only a subset of those model viruses in the end-of-life resin study. The two model viruses chosen, a retrovirus and a parvovirus, span a range of virus characteristics—a large, enveloped RNA virus and a small, non-enveloped DNA virus, respectively. If the clearance of these two viruses does not significantly change between unused and maximally cycled resin, then additional data from other viruses are not generated. This approach is supported by published literature and by historical data from Pfizer and elsewhere in the industry that show that clearance of other viruses will also remain unchanged.
For each resin (unused and maximally cycled), only one packed column is used for both model viruses, which also includes the replicate runs for each virus. Using this approach, the retrovirus, being the more sensitive virus, would be evaluated during the first set of runs. Once these runs are complete, the parvovirus, being less sensitive, would be evaluated in the final set of runs. Each column run would, as part of the process, include a complete cleaning and sanitization prior to its next use. This approach also allows for carryover analysis to be performed for each virus run. The overall design and flow of the study is shown in Figure 7. The results from this type of study for one project at Pfizer were presented at the Cambridge meeting and are shown in Table II.
Flow diagram of alternative approach to end-of-life virus studies.
Results of Alternative Approach to Viral Clearance/Carryover Studies on AEX Resin
As shown in Table II, there was no carryover of virus observed (below the limit of quantitation, or LOQ) between runs of the same virus or between the two virus types that were tested. The negative carryover results demonstrate this is an acceptable approach to evaluate viral clearance as part of an end-of-life resin study. These results also further demonstrate the other point to be made from these data—there was no difference in virus carryover or viral clearance capacity between unused (new) and maximally cycled resin. In addition to these results, an example published data set from industry (10⇓–12) was also shown (Table III) that further confirms the consensus opinion from the biotechnology industry that, for well-characterized chromatography resins, there has been no significant change observed in viral clearance capacity between unused (new) and maximally cycled resin. Consequently, these types of studies on end-of-life resins should no longer be considered required. For new-to-the-market and less-characterized chromatography resins (e.g., new ligands or modes like mixed-mode), the study design presented herein offers an efficient means to evaluate viral clearance for end-of-life resin.
Compilation of LRVs from the Public Domain
Virus Clearance with Chromatography Media Reuse for a mAb Process (E. Wilson; GSK)
GSK's mAb A is a Chinese hamster ovary (CHO) cell–expressed IgG1 mAb. The mAb A purification process includes three chromatography steps: Protein A affinity chromatography, AEX chromatography in flow-through mode, and HIC. A small-scale chromatography resin reuse study was done for the three steps to evaluate the effect of reuse on product quality and process performance and was presented at the Cambridge meeting. Virus clearance was claimed for two of those steps based on previous studies with new chromatography media. Virus clearance after reuse was evaluated for those steps with a panel of four model viruses: X-MuLV, porcine parvovirus (PPV), PRV, and Reo-3. Because initial clearance results were needed for business reasons before reused chromatography media were available, the new and cycled spiked experiments were not done concurrently. However, the experiments were done at the same laboratory, with the same load material and virus grade (but not necessarily the same virus lot), and with a three to five month gap between the new and cycled experiments. The number of reuses was based on the manufacturing facility request for a set number of batches, with the total reuses dependent on the number of cycles per batch for each step.
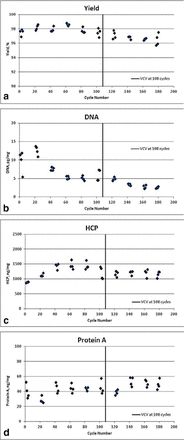
Protein A chromatography: Product quality and process performance were evaluated with a qualified small-scale model using a 1.1 cm diameter column. Initially, 108 cycles were completed to meet minimum manufacturing requests, and then reuse continued to 180 cycles. The small-scale model matched production-scale packed bed height, linear flow rate, buffer composition and volume, peak collection, and cleaning regime (every second cycle). Load material was sourced from one production-scale lot. Load ratio was set at the upper end of normal operating range. Quality (e.g., Protein A, DNA, host cell protein [HCP]) and process performance (e.g., yield, eluate volume) attributes were evaluated for the first four cycles of every 20 cycles. Later cycle sets were compared to the initial cycle set and reuse was found to be acceptable up to the 180 cycles evaluated. Figure 8 shows selected attributes such as antibody yield, DNA, HCP, and Protein A.
Attribute data at reuse of Protein A resin. (a) antibody yield, (b) host cell DNA, (c) HCP, (d) Protein A.
For virus clearance, a 2.2 cm diameter column was cycled 108 times, unpacked, and then repacked in four 1.1 cm diameter columns. The parameter settings were the same as the nonspiked reuse experiments with the qualified small-scale model. A 2% spike of virus stock was added to the load. The resulting LRV was compared to the LRV for earlier duplicate experiments with new chromatography media using the same small-scale model.
There was no reduction in virus clearance on Protein A with reuse up to 108 cycles. There was an LRV increase of greater than 1 log10 for X-MuLV and PRV (Table IV). While product quality and process performance were acceptable at 180 reuses, the maximum was set at 108 based on the reuses tested for virus clearance.
Viral Clearance on New and Cycled (>108) Protein A Chromatography Media
AEX chromatography: Product quality and process performance were evaluated for 54 reuses with a qualified small-scale model using a 0.5 cm diameter column. The small-scale model matched production-scale packed bed height, flow rate, buffer composition and volume, peak collection, and production-scale cleaning regime (every cycle). Load material was sourced from multiple production-scale lots. Quality (e.g., DNA, purity) and process performance (e.g., yield, product volume) attributes were evaluated for the initial and final five cycle sets and reuse was found to be acceptable at 54 cycles.
For virus clearance, a 1.1 cm diameter column was cycled 54 times, unpacked, and then repacked in four 0.5 cm diameter columns. The parameters were set to match the parameters for the nonspiked reuse experiments with the qualified small-scale model. Load material was sourced from one production-scale lot. X-MuLV and PRV stock were spiked at 2%, and PPV and Reo-3 were spiked to a target total virus load. The resulting LRV was compared to the LRV for earlier duplicate experiments with new chromatography media using the same small-scale model (Table V). For the AEX step, clearance for new and reused resin was within ∼1 log10 of LRV. The maximum number of reuses was set at 54 based on product quality, process performance, and virus clearance.
Viral Clearance on New and Cycled (>54) AEX Chromatography Media
For both chromatography steps, the lowest LRV of the three experiments was used for cumulative clearance calculation.
AEX Resin Aging for Viral Clearance Studies (Richard Chen; Eli Lilly)
AEX chromatography is a critical unit operation in the Eli Lilly Indiana purification platform from both a process impurity and viral clearance perspective. Since the unit operation is performed in flow-through mode, AEX resin aging is assessed during viral clearance studies. At the Cambridge meeting, Lilly presented a study where resin was aged beyond the expected column lifetime in manufacturing (90 cycles) using two different methods.
The first aging method was product aging (Figure 9a). A small-scale column was repeatedly exposed to product, process buffers, and clean in place (CIP) buffers (acetic acid + sodium hydroxide). Column performance (chromatographic profiles and yields) and product quality (purity and process impurity levels) were periodically monitored throughout the study. No change in column performance or product quality was observed after 90 cycles. After the product aging study was completed, the column was used for a subsequent viral clearance study.
Approach for AEX product aging study (a) and AEX CIP aging study (b).
The second resin aging method was CIP aging (Figure 9b). A larger-scale column was exposed to primarily CIP buffers. A worst-case, longer contact time was implemented for every CIP cycle. A product cycle (product, process buffers, and CIP buffers) was run at the beginning, middle, and end of the study to assess column performance and product quality after this regime. Column performance and product quality were comparable between all three product runs After the CIP aging study was completed, the column was unpacked and the resin was used to pack several smaller-scale columns for viral clearance studies. Both new and aged AEX resins were evaluated for clearance using a panel of four model viruses. This strategy was used for three different mAbs (molecules A, B, and C).
Viral clearance results for the three separate mAbs are summarized in Table VI. X-MuLV log reduction factors (LRFs) were comparable between new, product-aged, and CIP-aged resins across three different molecules. Robust AEX clearance was observed in all cases. In addition, no significant LRF trends were observed between new and CIP-aged resins for MVM, PRV, or BVDV across three different molecules.
Viral Clearance Results for Molecules A, B, and C
The AEX viral clearance strategy described was successfully executed to support marketing authorization applications for three separate mAbs. One of the antibodies has already been approved by regulatory authorities (US, EU, Japan, and other countries). Proper planning and scheduling was a key factor for successful execution of the AEX viral clearance studies due to study linkages and longer duration times of precursor studies (e.g., resin aging).
Impact of Cycled Chromatography Resin on Viral Clearance (K. Cai; MedImmune)
Chromatography resins can be reused for commercial manufacturing within validated column lifetime/cycle limits. A typical validation approach is to perform a small-scale study demonstrating consistent process performance over time up to a target cycle number. The column lifetime limit for commercial manufacturing is then conservatively assigned with a 10% safety margin. A viral clearance study is subsequently performed using the cycled resin in a side-by-side comparison with new resin.
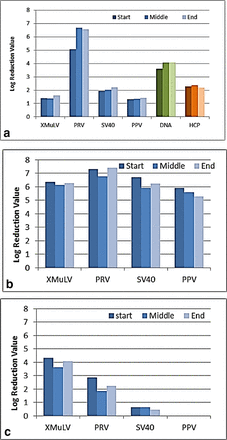
MedImmune presented a column lifetime study of a bind-elute CEX chromatography step for a mAb purification at the Cambridge meeting. In the study, consistent performance was demonstrated up to 115 cycles (data not shown); based on this the column lifetime limit, the CEX column was designated as 104 cycles. A viral clearance study was carried out at cycle 2 (start), cycle 56 (middle), and cycle 121 (end) using four model viruses: X-MuLV, PRV, SV40, PPV. All experiments were performed in duplicate with the lower virus LRVs reported. DNA and HCP clearance data obtained during column cycling are also reported for reference (Figure 10a). For the same purification process, similar studies were carried out for a flow-through AEX chromatography step (Figure 10b, lifetime = 108 cycles, end viral clearance at 123 cycles), and a bind-elute hydroxyapatite chromatography step (Figure 10c, lifetime = 28 cycles, end viral clearance at 33 cycles).
Viral clearance across resin lifetime for chromatography steps. (a) CEX chromatography, (b) AEX chromatography, (c) hydroxyapatite chromatography.
These results demonstrate that any difference in LRV for each virus between new and used resin is typically small. Occasionally MedImmune has observed that used affinity chromatography resin provides a better LRV compared to new resin (data not shown), a result consistent with published literature. Overall, our experience suggests that viral clearance capability is maintained, or is not negatively affected, by resin age within lifetime limits established based on process performance outputs.
Summary
Guideline ICH Q5A (1) recommends to consider virus clearance capacity during the reuse of a column. It is currently wide practice to perform product-specific virus clearance studies using new chromatographic resin as well as recycled resin for marketing/licensing applications. There is a perception that such studies alone can address this issue and are expected by regulators. However, the guideline has never been worded in such a strict sense and in ICH Q5A it is sated: “Over time and after repeated use, the ability of chromatography columns and other devices used in the purification scheme to clear virus may vary. Some estimate of the stability of the viral clearance after several uses may provide support for repeated use of such columns.” It is not required to perform studies with reused resin in each case. In this session an impressive amount of data, accumulated from many viral clearance studies with used and reused resin, was presented. The majority of data (with very few exceptions) showed consistent virus reduction over the resin lifetime. In cases where different LRVs are obtained, nuanced interpretation with caution is warranted if the studies are not directly performed side by side, as variation in the complex experimental setup might introduce some variation. However, the significance of such variation, if observed, has to be assessed in the context of the overall viral safety goals and specific viral clearance strategies. The aim of the cycled resins studies described above was to assure that there is no or only minimal detrimental impact of cycling and resin reuse on viral clearance, not to measure precise “before and after” LRVs. Subsequent to the original publication of ICH Q5A in 1998, specific virus clearance steps such as detergent treatment or virus filtration have been increasingly introduced for many products. Considering the overall clearance capacity in the context of a multistep manufacturing scheme, a certain variability of chromatographic purification steps over the lifespan of the resin could be considered acceptable, while it might be more critical if a chromatography was a key step in a more abbreviated process.
Performing studies with used and reused resin with multiple (three to four) model viruses for each marketing/licensing application can require a significant workload, process materials, and resources. Thus, the possibility to reduce the workload and material used for such studies was intensively discussed at the Cambridge meeting. Although, recycled resin for these studies is usually produced at small scale in a model of routine manufacturing, a significant amount of material and time is needed to cycle the small-scale model and volume of process fluid as feedstock (as well as buffer) to produce a sufficient amount of resin with 50 to 300 cycles of reuse for qualification studies. For example, if a single cycle in a typical large-scale process takes 5 h from equilibration to cleaning/regeneration, then 300 cycles run at small scale just to prepare the resin would take 1500 h (about two months) if the lab scale chromatography system was programmed to run nonstop. If 1.5 L of harvest feedstock per cycle was required to prepare resin for a small-scale protein A model unit operation, then 450 L of harvest (a medium-sized bioreactor) would be needed to get to that stage (assuming no wastage). Aside from buffer and feedstock consumption, bioburden control in a lab chromatography system running for two months nonstop is a challenge. The time taken for completion of studies will be longer if a large panel of viruses is used.
These scenarios can be mitigated somewhat if the same aliquot of recycled resin is used for a single virus clearance study, where three to four viruses are added as a “multi-spike”. This can be an appropriate way of reducing the amount of resin needed for such studies, provided that the virus detection systems do not interfere with each other. Another alternative, acceptable strategy would be to perform such studies with the same aliquot of resins in sequence, for example reduction of X-MuLV is studied with resin cycled 50 times, reduction of SV40 is studied with resin cycled 51 times, and reduction with parvovirus using resin cycled 52 times. At the Cambridge meeting, it was further suggested to generally limit the number of model viruses in cycling studies to two (i.e., X-MuLV and a parvovirus). It is not possible to conclude which of the viruses represent the worst case and this will be dependent upon the specific process. For example, reduction of BVDV by nonbinding AEX chromatography was less than that of animal parvoviruses (see contribution from R. Chen above). Nevertheless, these two model viruses have been generally accepted as sufficient for clinical-trial applications using well-characterised cell substrates such as CHO cells. A further approach to save material needed for producing recycled resin was presented by R. Chen. He compared the viral clearance of product-aged resin and CIP-aged resin where an AEX resin was exposed to the sanitization agents and buffers only. No significant difference in virus reduction was observed with X-MuLV while the other model viruses were investigated with CIP-aged resin only. This strategy was successfully applied for licensing in the US, EU, Japan, and other countries. CIP and buffer costs are lower than process fluids such as bioreactor harvests, thus this approach affords considerable process economic advantages.
For protein A chromatography, the experience presented in this session indicated a trend toward slightly higher LRVs with reused protein A resin. Such a trend has also been observed before (4), and has been suggested to be associated with a phenomenon of “conditioning”. Interaction of X-MuLV particles and mAbs and/or the resin has been suggested to explain the co-elution of some virus with antibody during Protein A chromatography (13). Therefore, decreasing antibody-binding capacity of cycled resin or blocking of nonspecific binding sites for virus might explain the trend for slightly increasing LRVs. The Center for Drug Evaluation and Research (CDER) considers virus reduction by Protein A chromatography robust with respect to resin age and has developed internal guides for reviewers to this effect. At the European level, studies with cycled resin are currently expected and usually provided at marketing authorization, although there have been few specific cases where studies with used resin were performed postapproval after completion of full-scale production runs in order to confirm the expected virus clearance.
Most studies on AEX chromatography presented at this meeting showed comparable virus reduction with new and reused resin. In the case of nonbinding mode, where virus and other impurities such as negatively charged proteins or host cell DNA are bound to the resin while the product itself flows through, the viral clearance was robust provided that the integrity and binding capacity of the resin was not affected. It remains challenging to identify a single suitable surrogate (e.g., break-through of impurities, pressure rise, or loss of product yield) that is applicable for all the various brands of resin and applications that reliably can predict sufficient viral clearance. AEX resins are often sanitized by NaOH. It would be useful as a followup to the meeting to conduct experiments where virus is spiked to various concentrations of NaOH to find the virus inactivation failure point for stable viruses such as parvoviruses. Studies using high concentrations were presented at this session and are in line with earlier observations. NaOH reliably inactivates all viruses tested so far, but the minimum concentration for universal virus inactivation has not yet been determined. Only at low concentrations (≤0.3M) do the inactivation kinetics become biphasic and some residual infectivity of non-enveloped viruses such as animal parvoviruses or reovirus could be detected after treatment of virus with NaOH. Such a resistant fraction could represent aggregated virus particles, and this could be an issue if a column is cleaned using a low-concentration sanitization buffer. Thus, while it seems difficult to speculate about the aggregation status of a virus contaminant in a given specific intermediate it would be desirable to elaborate a minimum NaOH concentration that reliably inactivates resistant model viruses such as parvoviruses. Given reliable inactivation this could justify not performing resin carryover studies and is a reasonable follow-up action item from the Cambridge meeting.
- © PDA, Inc. 2016