Abstract
Use of prefilled syringes to self-administer biologics via subcutaneous administration provides convenience to patients. The barrel interior of prefilled syringes is typically coated with silicone oil for lubrication to aid plunger movement at the time of administration. This study intended to evaluate the impact of formulation variables on the silicone oil on the barrel interior surface. Characterization techniques including syringe glide force, break loose force, Schlieren imaging, contact angle, inductively coupled plasma spectrometry, and thin film interference reflectometry were used in assessing the interactions. Data indicated that formulation variables such as pH, buffer/tonicity agent type and concentration, and surfactant present in the formulation can effect silicone oil lubrication of prefilled syringes, leading to changes in functional properties of the syringe over time. Syringe samples containing acetate and histidine buffers showed an increase in glide force at accelerated storage temperature conditions, but the change was minimal at 5 °C. The samples with the highest glide force correlated with the presence of mannitol in combination with sodium acetate buffer. Sodium chloride had lesser impact on glide force than mannitol. Samples with higher glide force exhibited a substantial change in the silicone oil layer of the syringe, as observed with Schlieren imaging, as well as a significant reduction in surface hydrophobicity, as demonstrated through contact angle measurement. These data indicated that the structure of the siliconized surface can change over time in contact with different formulations. During formulation development of drug products in prefilled syringes, in addition to potential impact on molecule stability, the selection of formulation variables should also be guided by assessing the impact to syringe functionality with the glide force as one of the key parameters.
LAY ABSTRACT: Self-administering drug products packaged in prefilled syringes provides convenience to patients. The interior of a prefilled glass syringe is typically lubricated with silicone oil for easy plunger movement during injection. This article discusses the impact of formulation excipients on silicone oil coating inside the syringe. Characterization techniques were used to assess the ease of plunger movement and structure of the silicone coating. Data indicate formulation excipients can affect silicone oil distribution of prefilled syringes, leading to an increase in plunger glide force at accelerated storage temperature conditions. The increase in glide force within a prefilled syringe with or without an auto-injector can have an impact on dose accuracy and user experience. Syringes with a higher plunger glide force appeared to exhibit a change over time in surface energy and structure of the silicone oil layer in contact with particular formulations.
- Formulation development
- Buffer
- Semi-finished
- Prefilled syringe
- Functionality
- Break loose force
- Glide force
- Schlieren imaging
- Contact angle
- Monoclonal antibodies
- Silicone oil
- Inductively coupled plasma emission spectroscopy
- Thin film interference reflectometry
Introduction
With a larger number of therapeutic monoclonal antibodies (mAbs) under development, drug administration using prefilled syringe systems have become increasingly important, especially for those that require chronic administration (1). The primary factors driving the growth of prefilled syringes over two-step vial syringe processes include ease of administration and convenience for health care professionals and patients (2, 3). In addition, the use of prefilled syringes provides for higher dose accuracy and reduced overfill requirements. Administration by subcutaneous (SC) route is one of the most preferred choices to deliver therapeutic proteins, especially for frequent treatments, long-term regimen, or self-administration by patients. Particularly, combination products such as prefilled syringes and auto-injectors enable patient self-administration, shifting the point of care from hospital to patient's home (4).
Auto-injectors can be very effective in enhancing user convenience while also driving a competitive advantage (5). The interior surface of a prefilled syringe is typically coated with silicone oil for lubrication to aid plunger movement during use. Prefilled syringes with less than a nominal amount of silicone oil may lead to a higher break loose force or glide force for manual syringes, and longer injection time or even stalling of auto-injectors. Because silicone oil is sprayed onto the syringe interior surface without chemical bonding, it was reported silicone oil in empty syringes can migrate over time during storage (6). Another study examined the effect of different process parameters of silicone oil coating, including curing temperature, applied amount, and viscosity of silicone oil, on the physical stability and leaching of silicone coating exposed to aqueous medium (7). Most literature on silicone oil in syringes has been focused on the stability of mAbs due to undesirable interactions between hydrophobic silicone oil and protein molecules (8⇓–10). However, there has been no report so far to the authors' knowledge evaluating the change in lubricity of silicone oil in prefilled syringes exposed to various protein formulations and subsequent impact on syringe functionality. In this work, we present the results of a statistically designed study to assess the impact of formulation variables such as pH, buffer species, tonicity agent, and surfactant concentration on the functionality of prefilled syringes over time. In order to assess the impact of only formulation excipients on syringe functionality, the study used “placebo” solutions without any active ingredient. Each placebo solution was prepared using a predetermined recipe, filled into a 1 mL long syringe, and placed on stability at 5 °C, 25 °C, and 40 °C. The assessment included syringe break loose force and glide force for syringe functionality, inductively coupled plasma emission spectroscopy (ICP) measurement of solutions for silicone concentration, and Schlieren and thin film interference reflectometry characterizations of syringe surface for silicone oil distribution and surface hydrophobicity of syringe interior as measured by contact angle.
Materials and Methods
Materials
The placebo solutions (Table I) were prepared, filtered (0.2 μm), and filled (1 mL) into glass syringes and plungered via mechanical insertion using an Inova SV122 syringe filler with an Optima OP45 peristaltic pump. The syringe filling operation was conducted under laminar air flow. All chemicals used in this study were USP grade. The sterilized glass syringe system was a BD Medical, Pharmaceutical System (BD) Neopak 1 mL long siliconized glass syringe with a staked needle (27G STW) closed with a West Pharmaceutical Services NovaPure W4023/50 bromobutyl Gray FluroTec® B2 coated (cross-linked silicone coating) plunger. The syringes and plungers were received as ready-to-use (RU) components from the manufacturer (washed, siliconized, and sterilized). A single batch of syringes and plungers was used for the study to minimize component lot-to-lot variability, including silicone oil levels and distribution. The syringe barrel lot for the study was selected based on thin film interference reflectometry data indicating the relative uniformity of silicone oil distribution.
Prepared Placebo Solutions (55 runs total)
Methods
The placebo-filled glass syringes (prefilled syringes) were tested by the following methods:
The Zwick/Roell Tension Compression Material Test Stand equipment (Model#: BT1-FR2.5TS.D14) was utilized to evaluate prefilled syringe functionality. The test method measures peak break loose force and maximum and average glide force (sustaining force) as the syringe plunger is pushed through the syringe barrel using a 100 N load cell to expel the syringe contents. The cycle speed was 9 in/min with an end-of-test force limit of 50.1 N. TestXpert II v3.4 software was utilized with the Zwick. A sample size of n = 10 syringes was used for each condition in glide force and break loose force testing.
Schlieren imaging (ZebraSci Satellite System) characterizes the distribution of lubricant of the interior of empty or filled syringes and other containers. Schlieren imaging was utilized in this study to visualize the distribution of silicone in placebo-filled syringes. A vision inspection with a telecentric lens was completed with structured lighting to provide a wavy backlight, enhancing the edges of silicone droplets back to the camera system. Multiple views of the syringe were captured and evaluated to complete the analysis (image stitching). A sample size of n = 6 syringes was used for each condition in Schlieren characterization.
Inductively coupled plasma optical emission spectroscopy (ICP-OES) was used to measure the silicone oil content from the empty, dried syringe after the buffer solution was drained. One and a half (1.5) mL of methyl isobutyl ketone (MIBK) with 5 ppm of indium was added to the empty syringe and allowed to rest for a minimum of 5 min. The syringe contents were then poured into 15 mL polypropylene tubes. The MIBK extraction was repeated for a total of three times for each syringe sample. An additional 7.5 mL of MIBK/5 ppm indium was added for a final dilution factor of 12×. The samples were mixed by inversion prior to analysis. A Varian Vista Pro with SPS-3 Auto-sampler ICP-OES was calibrated with 1.0 to 40 ppm polydimethylsiloxane (PDMS) standards. The method utilized a dual pass spray chamber and 0.8 mm one-piece torch. The analysis of the extracted syringes was reported as “μg” of silicone per syringe. A sample size of n = 3 syringes was used for each condition in ICP testing.
Thin film interference reflectometry (RapID Layer Explorer UT Instrument) with Layer Explorer software was used to measure the silicone oil thickness of empty syringes prior to and after formulation placebo exposure upon storage. The syringes were drained through the plunger end, rinsed with deionized (DI) water and vacuum dried at room temperature prior to testing. The method measures six lines with 175 points per line for a total line length of 35 mm. A sample size of n = 3 syringes was used for each condition in reflectometry characterization.
The contact angles of water droplets on emptied glass syringes were measured. The prefilled syringes were cut using a rotary cutting tool while containing formulation solutions to minimize glass residues generated during the sample preparation. The pieces cut from syringes were rinsed using DI water to remove buffer components left on barrel surfaces, then dried by nitrogen. Drops of MilliQ water (4 μL) were placed on the cut syringes. Using a Krüss Drop Shape Analyzer DSA100 instrument with Drop Shape Analysis 4, v2.1 software utilizing a circle analysis model, replicate video measurements (2.5 frames/s for 10–20 s) were completed. The final contact angle results represented an average of five stable frames over approximately 9 s. A sample size of n = 3 syringes was used for each condition in contact angle measurement.
Statistical Study Design
The following variables for the placebo formulation were selected for the experimental study design. The types of buffer and concentrations were selected based upon the ionization constant and buffer capacity of the buffer species over the pH ranges of 4 to 7, where the majority of the mAb formulations are typically formulated for optimal chemical and physical stability. Selection of tonicity agents in this study was based upon the common use of these excipients in mAb formulations for stability and iso-tonicity considerations. Since polysorbates are the most commonly used non-ionic surfactants for biologics, polysorbate 80 (also called PS80) at a reasonable concentration range was included in this study design. In addition, these variables were considered to have potential impact to silicone integrity on the syringe barrel based on literature reports and first principles understanding:
Buffer type (three levels—citrate, histidine, acetate)
pH
acetate: 4.5, 5.0, 5.5
histidine and citrate: 5.5, 6.0, 6.5
Buffer concentration, mM (two levels—low [10 mM] and high [20 mM])
Buffer solution pH (three levels specific to each buffer [See Figure 1])—low, mid, and high
Tonicity agent (two levels—sodium chloride and mannitol)
Tonicity concentration/ionic strength (two levels specific to each tonicity agent [See Figure 1])—low and high
Polysorbate 80 concentration, % w/v (two levels—low [0.02] and high [0.06])
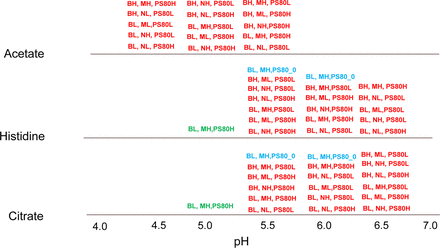
Statistical study design overview. BH = buffer high (20 mM); BL = buffer low (10 mM); MH = Mannitol high (5% w/v); ML = Mannitol low (2.5% w/v); NH = NaCl high (150 mM); NL = NaCl low (75 mM); PS80H = Polysorbate 80 (PS80) high (0.06% w/v); PS80L = PS80 low (0.02% w/v). Red type = main study factors, blue type = no PS80, green type = very low pH level (refers to histidine and citrate buffer species).
The statistical design selected (Figure 1) was a nearly orthogonal array design, which consisted of 48 of the 144 combinations, or one-third of the total number of possible combinations (indicated in red type in Figure 1). With this type of design, for the projection of any pair of variables there is balance at each of the combination levels in the number of trials performed. For example, for each of the six combinations of buffer (three levels) and buffer concentration (two levels), a total of eight runs were performed at each combination.
The study had 55 runs in total, as shown in Figure 1, which included seven additional runs performed based upon the specific interest to investigate certain parameters in greater detail. Five additional runs were performed without polysorbate 80 for specific variable combinations of interest (indicated in blue type in Figure 1). In addition, two runs were performed for histidine and sodium citrate buffers at pH 5.0 to examine the syringe functionality at low pH levels for the buffer conditions (indicated in green type in Figure 1) and also to evaluate the effect of buffer type formulated at the same pH. Each of the 55 runs were evaluated at three different storage conditions, 5 °C and 25 °C for up to six months, and 40 °C for one month.
The glide force and break loose force were obtained for ten syringes at each combination of stability condition and time point. The average of these ten values was computed and evaluated for evidence of trends due to one or more of the studied variables.
Results and Discussion
Glide Force and Break Loose Force
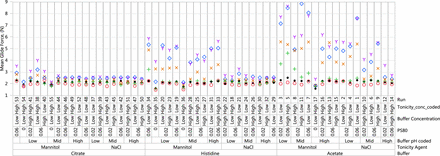
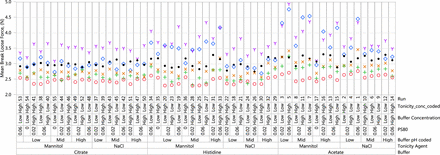
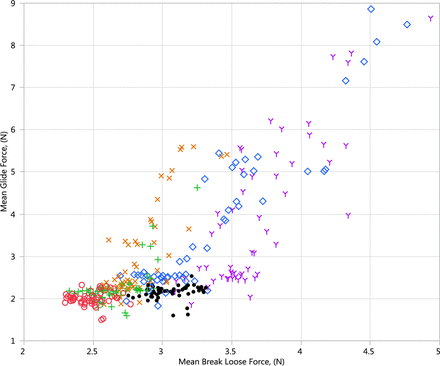
An understanding of the impact of formulation pH and excipients on syringe functionality begins with the two most important properties of device functionality, glide force and break loose force. The mean glide force results are presented in Figure 2. The break loose force data are presented in Figure 3, and the glide force versus break loose force profile is presented in Figure 4.
Mean glide force (N) results: acetate, histidine, citrate. Red circle = Initial; Green plus = 1 M (25 °C); Blue diamond = 1 M (40 °C); Orange x = 3 M (25 °C); Purple Y = 6 M (25 °C); Black dot = 6 M (5 °C).
Mean break loose force (N) results: acetate, histidine, citrate. Red circle = Initial; Green plus = 1 M (25 °C); Blue diamond = 1 M (40 °C); Orange x = 3 M (25 °C); Purple Y = 6 M (25 °C); Black dot = 6 M (5 °C).
Glide force versus break loose force results (N). Red circle = Initial; Green plus = 1 M (25 °C); Blue diamond = 1 M (40 °C); Orange x = 3 M (25 °C); Purple Y = 6 M (25 °C); Black dot = 6 M (5 °C).
As shown in Figure 2, glide force was affected by the storage condition, buffer type, pH, type of tonicity agent, and presence of polysorbate 80. The syringe samples containing sodium citrate buffer demonstrated a minimal change in glide force whereas samples containing histidine buffer showed a moderate change in glide force at accelerated temperature. The largest change in glide force was seen with syringe samples containing acetate buffer at the accelerated temperature. An increasing trend in glide force was seen for samples with histidine and acetate buffers at elevated temperatures, but the change was minimal at 5 °C up to six months. The highest glide forces were seen with syringe samples containing mannitol and polysorbate 80 with low to mid solution pH. All samples without polysorbate 80 had much lower glide forces regardless of buffer. Syringes exhibiting higher glide force increased further with long-term storage. Finally, the break loose force was more similar across different runs than glide force but still demonstrated similar trends of higher break loose force with samples containing acetate and histidine buffers, mannitol, and polysorbate 80 (Figures 3, 4).
The glide forces for these placebo syringes are in the 1–9 N range, which are relatively low to affect auto-injector performance; however, these data are obtained for placebo solutions in syringes with a viscosity of 1 cP. High-concentration mAb formulations with viscosities of 10 cP or higher will add a significant amount of hydraulic force to the overall glide force and will likely affect auto-injection time or dose delivery accuracy.
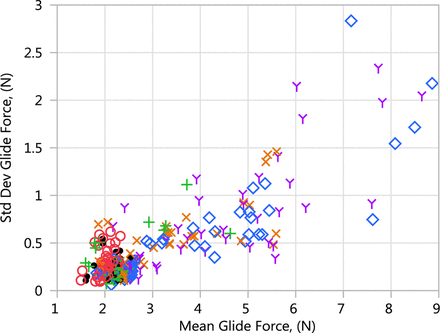
For each test performed, ten glide force and break loose force measurements were obtained. The averages are summarized in Figures 2⇑–4. Figure 5 shows the standard deviation of the glide force results plotted against the average of the glide force results for each combination tested. This figure shows that the variability of glide force increases as the average glide force increases. A similar pattern was observed for break loose force.
Standard deviation of glide force replicates versus average glide force results.
Statistical Summary
The average at each unique combination of stability condition and time point was initially evaluated separately to identify the important variables. At the initial time point, it was clear that there was little impact of the variables on the average glide force or break loose force. However, the impact of the variables became increasingly evident at 25 °C and 40 °C storage conditions as the range of the observed glide force and break loose force increased.
The variable called buffer type had a very significant effect and therefore this variable is shown at the lowest level. Other important variables included tonicity agent and buffer pH. While acetate buffer in general showed the largest increase in average glide force compared to the other buffers, when no polysorbate 80 was present, no increase in glide force was observed for an acetate buffer formulation.
The impact of storage temperature/time on stability can be observed in the variability chart. For variable combinations where there was little impact on glide force and break loose force, all of the results were similar. However, for variable combinations where there was an impact of storage condition and time, the results separate vertically.
Schlieren Imaging
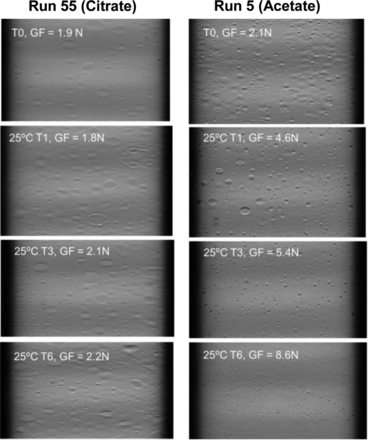
To understand the glide force and break loose force changes upon exposure to different buffer conditions, a nondestructive characterization technique of Schlieren imaging was conducted to evaluate syringe silicone oil distribution. Schlieren imaging is based on Schlieren optics principles to visualize the silicone oil in situ in the presence of formulation placebo solution (11). The representative Schlieren images for citrate (low glide force) and acetate (high glide force) placebo-filled syringes at initial and accelerated temperature of 25 °C up to six months are presented in Figure 6. The distribution and structure of silicone oil layer on the interior of placebo-filled syringes on accelerated stability correlated with the glide force results. Syringes with sustained lower glide force (e.g., citrate buffer without polysorbate 80) showed consistent surface structure within six months at least while syringes with increasing glide force (e.g., acetate buffer with polysorbate 80) exhibited a change in surface structure within the first month at 25 °C.
Representative Schlieren images for citrate and acetate placebo-filled syringes stored at 25 °C for up to 6 months (T0: initial, T1: 1 month, T3: 3 months, T6: 6 months, GF: glide force).
Thin Film Interference Reflectometry
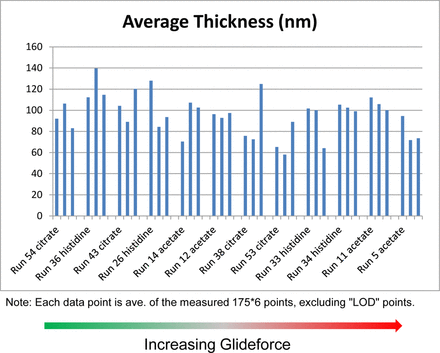
Based on Schlieren imaging and glide force data, it was hypothesized that a gradual erosion of silicone oil from the syringe surface might have happened leading to an increase in glide force for certain placebo formulations assessed in this study. Therefore, thin film interference reflectometry imaging was used to measure the thickness of silicone oil in the emptied syringe barrels of 12 selected 3 month 25 °C samples, which showed a contrast in glide force (see Table II for selected sample details). The syringes were emptied using a plunger rod to remove the plunger followed by draining liquid inside from the flange side. However, Figure 7 displays that reflectometry-measured silicone oil layer thicknesses were similar for all of the 12 selected syringe samples, suggesting the glide force change was not due to different silicone oil quantities on the syringes. This result supports another hypothesis that the silicone oil was not removed but rather altered to a different surface structure or energy state.
Selected Samples for Further Study Based on Range of Glide Force and Excipients
Reflectometry-measured silicone oil layer thickness.
ICP
To verify the reflectometry measurement of silicone oil layer thickness, ICP analysis of silicone extracted from the emptied syringe barrels was completed on the 12 selected 3 month 25 °C samples (Table III). Note ICP measurement was not conducted with the same exact syringes tested with reflectometry, due to the concern of reflectometry sample preparation (solution draining and water rinse) that may distort the ICP results. For new empty syringes, we found ICP and reflectometry results agreed well with each other in trending. The ICP data for these samples were consistent with reflectometry data, indicating that silicone oil was not removed from the syringe barrels by buffer exposure. However, these results are not in agreement with the Schlieren imaging or glide force data, due to the difference in what to measure with each of these analytical techniques. While both are optical techniques, the reflectometry method relies on thin film interference to deduce the thickness of the silicone oil layer, while Schlieren optics detects the refractive index gradient near the glass/silicone oil/water interfaces (11). The apparent change of silicone oil morphology observed via Schlieren optics suggested a change of silicone oil structure or adhesion to glass leading to a refractive index change. The change of silicone oil structure or adhesion may affect its lubricity and syringe functionality such as glide force, even without a change of total silicone oil amount on the surface.
ICP Silicone Results Ranked by Increasing Glide Force
Contact Angle Measurements and Schlieren Images for Citrate and Acetate Placebo-Filled Syringes
In order to assess if there is a relationship between glide force and surface energy, contact angle measurements were conducted on syringes that had been filled with different placebo formulations and placed on accelerated stability. The contact angle results for the citrate (low glide force) and acetate (high glide force) buffer-containing syringe samples are presented in Table IV. The contact angle data for other conditions are not included because these two conditions represent the low and high glide forces within the study design. The contact angle and Schlieren images for the citrate and acetate buffer-containing syringe samples are presented in Figure 8.
Contact Angle Results for Acetate and Citrate Syringe Samples
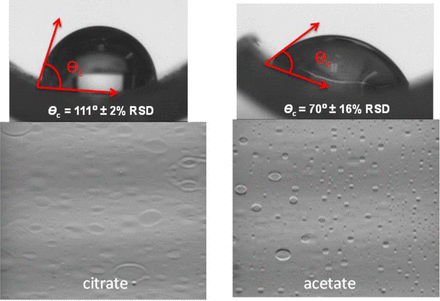
Representative contact angle of water droplet on drained and dried syringe surface and Schlieren images for citrate and acetate exposed syringe samples.
The contact angle results showed dramatically different hydrophobicity for syringes exposed to different placebo formulations with contrasting glide forces. The higher the contact angle, the more hydrophobic the surface is. In general, a surface with a contact angle higher than 90° is considered hydrophobic, and a surface with a contact angle lower than 90° is considered hydrophilic (14). The syringes exposed to citrate buffer with mannitol but without polysorbate 80 (lot 55) had a contact angle of 111°, which is consistent with the hydrophobic nature of the silicone oil coated surface (15). This result correlates well with the low glide force of the syringe indicating the lubricity of the silicone oil coating. However, the syringes exposed to acetate buffer with polysorbate 80 and mannitol (lot 5) showed a contact angle of 70°, indicating the hydrophilic nature of the surface. It is likely the silicone oil on acetate buffer-exposed syringes has undergone physical and/or chemical changes that rendered silicone oil less lubricious, resulting in higher glide force. Excipients such as polysorbate 80 may adsorb to the silicone oil coating on the syringe, as recently reported in a study indicating polysorbate 80 adsorption to silicone oil during long-term storage of mAb formulations in prefilled syringes (16), which can increase the hydrophilicity of the surface to reduce surface energy, consequently reducing the effectiveness of silicone oil lubricant.
For the two excipients (sodium acetate and acetic acid) in acetate buffer, sodium acetate was reported to have a severe effect on PDMS and acetic acid had only fair compatibility with PDMS (12). Polysorbate 80 as a surfactant can stabilize silicone oil emulsion droplets (8) and may facilitate removal of silicone oil from the surface into solution, but micro flow imaging (MFI) results (data not shown) did not indicate a higher number of subvisible or visible particles in the solution inside syringes containing polysorbate 80, nor did reflectometry or ICP data indicate the loss of silicone oil from the syringe surface. It is likely that polysorbate 80 may adsorb onto and diffuse into the silicone oil, which reduces surface energy and weakens silicone oil adhesion to glass, and consequently leads to an increase in break loose force and glide force, and also shows a different surface structure under Schlieren optics. On the other hand, sucrose was reported to increase silicone oil coalescence rates (13) and destabilize silicone oil emulsion droplet. It is possible that other sugar alcohols such as mannitol may also have an impact on silicone oil emulsion stability. The interactions among formulation excipients such as acetate, polysorbate 80, and mannitol may lead to a synergistic effect on silicone oil stability and functionality.
Conclusions
The results from this study indicate that both the formulation excipients and pH can affect the silicone oil lubricant of prefilled glass syringes under accelerated storage conditions, leading to changes in the functional properties of a syringe system. Syringe samples containing acetate and histidine buffer showed the greatest increase in glide force at accelerated storage conditions, but the changes were minimal under refrigerated storage conditions for up to six months. The samples with the highest glide force also contained mannitol and polysorbate 80 with solution pH in the range from 4.5 to 5. Samples with higher glide force appeared to exhibit a change in structure of the silicone oil lubricant of the syringe as observed by Schlieren imaging. While reflectometry and ICP data did not show a loss of silicone oil from the syringe surface upon exposure to acetate-containing solutions, the contact angle measurement showed a significant decrease in hydrophobicity of acetate-exposed syringe surfaces, leading to high glide forces reflecting the reduction of surface lubricity.
The data from this study suggest that in addition to assessing the impact of formulation variables on chemical and physical stability as part of combination product development, an assessment of the impact of formulation factors on syringe functionality should also be conducted to ensure acceptable dose-delivery performance.
Conflict of Interest Declaration
The authors declare that they have no competing interests related to this article.
Acknowledgments
The authors would like to acknowledge Peter William Sargent, Jr., Richard Meury, Daniel Mansell, Deirdre Delph, Bianca Smith, Craig Ferguson, Deana Furrey, Michael Berger, Ross A. Allen, Dr. Zhicheng (Patrick) Xiao, Jonathan G. Parker, Matthew J. Moon, Brian A. Finch, and Jessica Smith for their contributions to the study. The authors would like to thank Dr. Michael De Felippis for thorough review of the manuscript.
- © PDA, Inc. 2018