Abstract
Compendia methods have historically been used to assess heavy metals in both drug products and packaging material extracts. However, these methods have been found to be inadequate for elemental specificity and accurate measurements. The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) has published the Q3D, Guideline for Elemental Impurities, to provide a risk-based approach that specifies elements to be considered in a drug product risk assessment and permitted daily exposures (PDEs) depending on toxicological concern and route of administration. Consistent with these efforts, the United States Pharmacopeia (USP) withdrew the <231> Heavy Metals test procedure as of January 2018. The USP published new methods consistent with ICH Q3D risk-based approaches, <232> Elemental Impurities - Limits and <233> Elemental Impurities - Procedures. These new tests are intended for evaluation of drug products, leaving a gap in the assessment of extractable elements for packaging components. This gap prompted the need for a better understanding of the potential for elements of concern to extract from packaging materials and contribute to drug product elemental impurities. The present study investigated multiple extraction conditions coupled with modern analytical techniques to understand the capacity for elements to extract from elastomeric components. Most elements of interest, based on ICH or their potential for occurrence in elastomers, were ultimately recovered at levels below designated thresholds, allowing for correlation to PDE. These results highlight that although extractable elements from elastomeric components have the potential to contribute elemental impurities to a drug product, the actual contribution to cumulative levels would need to be calculated among all other potential sources as part of the process of elemental impurities assessment.
LAY ABSTRACT: Compendia methods have historically been used to assess heavy metals in final drug products and extracts from packaging materials. However, these methods were found to provide inadequate data to address the evolving risk concerns of elemental impurities in drug products and their potential toxic effects. The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use member countries are working toward implementing a risk-based approach that specifies elements to be considered in a drug product safety assessment and permitted daily exposures. The United States Pharmacopeia is coordinating with this goal by withdrawing the traditional procedure and replacing it with the tests that can inform safety risk assessments. However, the new tests are intended for evaluation of only final drug products, leaving a gap in the assessment of extractable elements for packaging components. The present study addressed this gap by focusing on elastomeric components used in injectable packaging systems and exploring appropriate elastomeric extraction methods coupled with modern analytical techniques to better understand the full potential for elements to extract from elastomers and contribute to the elemental impurity profile of a drug product.
- Elastomer
- Elemental impurities
- Extractables and leachables
- ICH Q3D
- Parenteral packaging materials
- USP <231>/<232>/<233>
Introduction
Elements in drug products may exist intentionally as part of the final product, or they may be unintentional impurities that could compromise medicinal quality and patient safety. An overall strategy for elemental impurity assessment should be based upon four key steps according to the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q3D, Guideline for Elemental Impurities: (1) identify known and potential sources of elemental impurities; (2) investigate potential for occurrence in a particular drug product; (3) compare observed or predicted levels of elemental impurities with established permitted daily exposures (PDEs); and (4) implement a control strategy to limit elemental impurities in the drug product (1).
Elemental impurities can arise from direct sources, such as inorganic excipients, or indirect sources, such as product contact materials that have the capacity to leach during drug product manufacture and throughout the product shelf life. Elements from packaging components have been shown to leach into drug products, resulting in a negative impact to product quality (2⇓⇓–5). There are several documented cases of elemental impurities leaching from packaging components into drug products. It was reported in as early as the 1980s that aluminum could leach from clay that is used as a filler in rubber closures (3). Shortly thereafter, the degradation of epinephrine in lidocaine HCl was found to be induced from leachable aluminum (6, 7). Metal cations, which can also originate from rubber, have been shown to cause protein conformational changes that can affect product quality and safety (4). Extractable elements in rubber components can arise from inorganic catalysts, mineral fillers, colorants, curatives, and process aids. Examples of elements used as rubber catalysts include: Al, Hg, Li, Ni, Rh, Ru, Ti, V, and Zn (see Table I for chemical names) (8, 9). Mineral fillers, such as talc or clay, are mined materials and will be a mixture of elements, some of which may contain trace toxic elements of concern, such as Pb, As, and Cd, as well as nontoxic elements, such as Mn, that could affect product quality (10).
Study Analyte Elements and Corresponding Chemical Symbolsa
Risk identification for potential elemental impurities from packaging components can be surmised from previous knowledge, experience, supplier information, and extractable studies. Literature can also inform on elements that could be present in packaging materials; some particularly thorough reviews exists on the subject (11, 12). Multiple sources of information are useful to understand and target elements from various materials, but these cannot substitute for verification of type, level, and effect of a particular drug product. The global implementation of the ICH Q3D document spurred greater interest in potential elements that could leach from packaging systems. Pharmaceutical manufacturers and components suppliers alike are contending with the acquisition of appropriate information to mitigate risks. Significant challenges exist with assessing extractable elements and correlating this information with potential leachable elements within an established PDE. For example, it has been shown that element concentrations can vary depending on the extraction solutions, and the probability for elemental impurities can be wide-ranging (13). These challenges highlight that while potential elemental impurity data from a packaging supplier can be useful to understand the capacity of each packaging component in order to contribute toxic and nontoxic elements, neither material composition nor empirical extractable data are necessarily predictive of potential leachables in a particular drug product. It is also not practical to assume an extraction study will capture all potential elemental impurities of concern, especially those present at trace levels, because the extraction conditions do not replicate the physicochemical interactions and storage conditions of a drug product.
Despite demonstrated capacity for packaging components to contribute to an elemental impurities profile, current guidelines do not adequately detail evaluation of potential elemental contribution from packaging materials. The withdrawn United States Pharmacopoeia (USP) method <231> Heavy Metals is currently referenced in the packaging chapters, <661> for plastics and <381> for elastomers, leaving a gap for the better understanding of extractable elements that would have the potential to migrate into drug products (14). To this end, the aim of the present study was to use modern methods to characterize rubber materials for the capacity to contribute elemental impurities but not to simulate for actual use.
Guidance on Elemental Impurities Assessments
Various compendia and standard documents exist to aid in the identification, reporting, and control of heavy metals. Since the implementation of the ICH Q3D document in 2015, there has been a major shift from the previous testing for heavy metals and limits.
ICH Q3D advocates a risk-based approach for assessment of elemental impurities in drug products and includes defined elements, limits, and PDE calculations. The publication of this guideline was instigated, in part, by a 2008 proposition by the USP to replace its longstanding test <231> Heavy Metals (15, 16). The method for detecting heavy metals in this test was based on water extractions with sulfide precipitation relative to a lead standard. The heavy metals method was neither elemental-specific nor sensitive enough to detect and recover most elements of concern. Similar heavy metal methodology was also used by the Japanese, European, and Chinese Pharmacopeias (17⇓–19). These testing strategies limited the ability of the method to specify the elements of interest and accurately measure and recover the species that could pose a potential risk to patient safety or drug product quality. However, ICH member countries have now adopted the ICH Q3D risked-based assessments. The United States Food and Drug Administration (FDA), European Medicines Agency (EMA), the Japanese Ministry of Health, Labor, and Welfare (MHLW), and Health Canada have all issued specific guidelines for compliance with ICH Q3D. The USP has supported these compliance efforts by developing new tests for evaluation of elemental impurities to replace the <231> document, including <232> Elemental Impurities-Limits and <233> Elemental Impurities-Procedures (20, 21). These USP methods also center on a risk-based approach for assessment and control of elemental impurities in drug products, with elements stratified into risk classes according to route of administration, likelihood of occurrence, and PDE.
Study Design for Extractable Elements in Elastomers
This study focused on methods to acquire reliable data from elastomeric components to inform risk of drug product elemental impurities based on the presence of elements that could potentially leach into any drug product. A screening approach was used to capture the 24 elements of interest in ICH Q3D (excluding gold) as well as 11 other elements that may arise from packaging (Table I). Development of appropriate methodology was explored as sensitive; specific methods for assessing elemental impurities potentially arising from elastomers were not clearly defined at the time of the study. Exaggerated extraction conditions with multiple solvents were explored, with concurrent troubleshooting to ensure recovery and stability of specified elements through the extraction process and analysis by inductively coupled plasma mass spectrometry (ICP-MS). While not predictive for a specific use, the resultant data can inform on the elements that could possibly extract to drive more targeted analyses of final drug products. The methods developed can also be used to generate comprehensive elemental impurity profiles in order to support selection of elastomer components for intended use and corroborate the presence of elemental impurities in drug products.
Materials and Methods
Materials and Reagents
Chlorobutyl and bromobutyl stoppers (20 mm) were used for the study. To minimize potential metal contamination, plasticware was used throughout the extraction and analysis process, including centrifuge tubes (VWR 15 mL, metal-free) and volumetric flasks (Nalge Nunc International, Corporation, Rochester, NY, USA). Glass vessels can be composed of elements, such as Si, B, Al, Na, K, Ca, Mg, and Ba; these in turn have the potential to contribute the elements to an impurity profile (22, 23). The following reagents were used: nitric acid (HNO3) (PlasmaPure Plus, SCP Science, Baie-D'Urfé, Canada); purified water (resistivity ≥ 18.0 MΩcm, TOC ≤ 500 ppb); ammonium hydroxide (NH4OH) (BDH Aristar Plus, VWR International, Radnor, PA, USA); 32%–35% hydrochloric acid (HCl) (PlasmaPure Plus, SCP Science, Baie-D'Urfé); SmartTune ELAN DRC Plus II (PerkinElmer, Waltham, MA, USA); individual ICP standards at 1000 μg/mL, including Ca, Cr, Fe, Ge, Mg, Si, Sn, V, W, and Zn (Inorganic Ventures, Christiansburg, VA, USA); multielement standard with 100 μg/mL of Ir, Os, Pd, Pt, Rh, and Ru (SCP Science, Baie-D'Urfé, Canada); a custom multielement standard with 100 μg/mL of Al, Ag, As, B, Ba, Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Pb, Sb, Se, Si, Ti, Tl, V, and Zn (Inorganic Ventures, Christiansburg, VA, USA); a multielement internal standard with 10 μg/mL each of bismuth (Bi), holmium (Ho), indium (In), lithium-6 (6Li), scandium (Sc), terbium (Tb), and yttrium (Y) (SPEX CertiPrep, Metuchen, NJ, USA); and ultrahigh purity oxygen (Praxair, Inc., Danbury, CT, USA).
Preparation of Standard Solutions
A 100 ng/mL internal standard containing Bi, Ho, In, 6Li, Sc, Tb, and Y was prepared in 1% (v/v) HNO3. Standard calibration solutions were prepared in 2% (v/v) HNO3, 0.5% (v/v) HCl, and 200 ng/mL Au with varying elemental concentrations as outlined below:
Standard 1—0.05 ng/mL Hg; 0.1 ng/mL Ag, Al, As, B, Ba, Cd, Co, Cu, Ge, Ir, Mn, Mo, Ni, Os, Pb, Pd, Pt, Rh, Ru, Sb, Se, Sn, Ti, Tl, W, and Zn; 1 ng/mL Cr and V; and 5 ng/mL Ca, Fe, Li, Mg, and Si.
Standard 2—0.5 ng/mL Hg; 1 ng/mL Ag, Al, As, B, Ba, Cd, Co, Cu, Ge, Ir, Mn, Mo, Ni, Os, Pb, Pd, Pt, Rh, Ru, Sb, Se, Sn, Ti, Tl, W, and Zn; 1 ng/mL Cr and V; and 50 ng/mL Ca, Fe, Li, Mg, and Si.
Standard 3—2.5 ng/mL Hg; 5 ng/mL Ag, Al, As, B, Ba, Cd, Co, Cu, Ge, Ir, Mn, Mo, Ni, Os, Pb, Pd, Pt, Rh, Ru, Sb, Se, Sn, Ti, Tl, W, and Zn; 50 ng/mL Cr and V; and 250 ng/mL Ca, Fe, Li, Mg, and Si.
Standard 4—5 ng/mL Hg; 10 ng/mL Ag, Al, As, B, Ba, Cd, Co, Cu, Ge, Ir, Mn, Mo, Ni, Os, Pb, Pd, Pt, Rh, Ru, Sb, Se, Sn, Ti, Tl, W, and Zn; 100 ng/mL Cr and V; and 500 ng/mL Ca, Fe, Li, Mg, and Si.
The following calibration verification standards were prepared in the same manner as the standard calibration solutions:
2.5 ng/mL Hg.
5 ng/mL Ag, Al, As, B, Ba, Cd, Co, Cu, Ge, Ir, Mn, Mo, Ni, Os, Pb, Pd, Pt, Rh, Ru, Sb, Se, Sn, Ti, Tl, W, and Zn.
50 ng/mL Cr and V.
250 ng/mL Ca, Fe, Li, Mg, and Si.
Preparation of Elemental Spike Solvents and Solutions
Extraction solvents were prepared in a range of pH values to capture any potential effects of hydrogen ion concentration on elemental extraction and recovery (24, 25). Solvents were prepared by adding either HNO3 or NH4OH to purified water and adjusting to acidic (pH 2.5), neutral (pH 7), and basic (pH 9). The extraction solvents were modified on the basis of results to consist of 2% HNO3, 0.5% HCl, and 200 ng/mL Au for optimal analyte recovery and stability. A spike stock solution was prepared from a custom 100 μg/mL multielement standard, 100 μg/mL platinum group element standard (Ir, Os, Pd, Pt, Rh, and Ru), and 1000 μg/mL single-element ICP standards of Ca, Cr, Fe, Ge, Hg, Li, Mg, Si, Sn, V, and W. The resultant element concentrations in the spike stock solution were: 5000 ng/mL Ca, Fe, Li, Mg, and Si; 1000 ng/mL Cr and V; 100 ng/mL Ag, Al, As, B, Ba, Cd, Co, Cu, Ge, Ir, Mn, Mo, Ni, Os, Pb, Pd, Pt, Rh, Ru, Sb, Se, Sn, Ti, Tl, W, and Zn; and 50 ng/mL Hg.
Preparation of Extracts
For all experiments, three replicate preparations of elastomeric closures were weighed (nominal weight 3.5 g, surface area of approximately 20 cm2) in a Teflon microwave digestion vessel. Along with the elastomeric samples, three blank samples (one for each of the extraction solvents) were prepared. Select blanks and elastomer preparations were spiked with the appropriate solutions either before or after extraction as outlined in the Results section. For extraction, the sample vessels were sealed and subjected to heating at 120°C for 30 min in a CEM MARS 5 (CEM Corporation, Matthews, NC, USA) microwave digester with HP500 vessel assembly to simulate the autoclave conditions outlined for elastomeric extraction in the USP <381> document.
Analysis
All prepared solutions were analyzed by ICP-MS using a Perkin Elmer ELAN DRC II. Instrument conditions are detailed in Table II. The Dynamic Reaction Cell (DRC) mode of the instrument with forced introduction of ultrahigh purity oxygen was used to eliminate interference when necessary. The following system suitability criteria were evaluated: the calibration curve for all analytes (with a nonweighted linear fit) showed an R-value of not less than 0.995. The calibration standards were all 80%–120% of the prepared concentration. The blanks did not contain any analyte concentrations at a level greater than the limit of quantitation (LOQ). The percent recovery for the LOQ was 70%–130% of the prepared concentration.
ELAN DRC II ICP-MS Instrument Conditions
Results and Discussion
The primary objective of this study was to develop a methodology to enable characterization of elastomeric components used in injectable packaging systems and explore appropriate extraction methods coupled with modern analytical techniques to better understand the full potential for elements to extract from elastomers and contribute to the elemental impurity profile of a drug product.
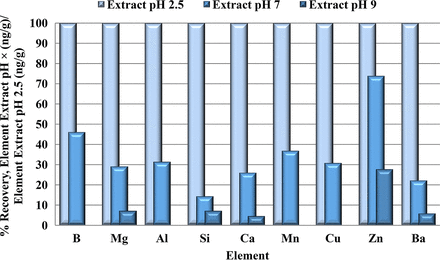
A series of experiments was performed to identify ideal extraction conditions that ensure maximal analyte recovery and method sensitivity. For the first experiments, the sample solvents were split in half post extraction, either spiked with the spike stock solution or left unspiked as shown in Table III, and analyzed 24 h later to determine stability of the 34 analytes in the different solvents. The three extraction solvents were analyzed with elastomeric components alone first to establish any potential solvent effects on recovery. The acidic solvent, purified water at pH 2.5, extracted a higher concentration of the detected elements (Figure 1). Extractions with all three pH aqueous solvents were then carried out with element spikes to ensure stability of elements post extraction. Without elastomeric components, recoveries for all spike elements were between 90% and 100% (data not shown). With elastomeric components, all spike elements were recovered within the acceptable calibrated range of 80%–120% with the exception of Al, Ba, Ca, Cu, Hg, Mg, Os, Si, Sn, and Zn (Table IV). Elements Al, Ca, Mg, Si, and Zn are commonly added to elastomers and can be present in variable levels from part to part, so the unspiked elastomer controls that the spiked elastomer samples were normalized to also had variable levels of these elements, resulting in wide-ranging recovery percentages. The variability also highlighted that the current extraction conditions may not be ideal for maximum recovery.
Preparation of Spike Solvents Post Extractiona
Comparison of extraction solvents with components only. All results were normalized to pH 2.5. Mg, Si, Ca, Zn, and Ba were detected in each of the three extraction solvents. B, Al, Mn, and Cu were only detected in the purified water pH 2.5 and purified water pH 7 extracts. Purified water at pH 2.5 extracted the highest concentration of the detected elements.
Spike Recoveries for Aqueous Extracts with Varying pHa
To increase overall potential for recovery in the second round of experiments, a number of adjustments were made. First, the solvent pH was decreased to exploit the greater solubility of metal ions in acidic solvents (26). Further, Au has been shown to stabilize Hg in solution without adding interference to ICP-MS analysis (27), so a single spike solvent was consequently prepared consisting of 0.5% HCl and 200 ppb Au in a 2% HNO3 solution. Additionally, elemental spikes were added before extraction to ensure stability of the analytes throughout the microwave extraction process. Li was also examined as an analyte in this second round, as like alkali metal Na or K, it could arise from mined materials. The modified acidic extraction solvent stabilized and increased the recovery of Hg to approximately 94%, increased the recovery of Cu to a range of 100% to 122%, and recovered most other elements of concern within the appropriate range. It was determined that this 2% HNO3/0.5% HCl/200 ppb Au extraction solvent would be ideal for element recovery from elastomers for subsequent extractions (Table V).
Spike Recoveries for Acid Extractsa
The extractions were then repeated for elastomeric components in the 2% HNO3/0.5% HCl/200 ppb Au extraction solvent with no element spiking to show suitability for evaluation of elastomeric components. Most elements were recovered at levels <0.05 μg/g threshold determined from ICH Q3D PDE for parenterals (Table VI). Elements that are often intentionally added to elastomers or have a higher likelihood of arising from elastomeric processing were detected, but levels were low despite the extreme extraction conditions. Mg and Si were exceptions, as these are known constituents of the formulation of the analyzed elastomers. Saturation of the detector by these two elements precluded the correlation of concentrations to PDE. Cumulatively, these results show that this method provides a tool to reliably recover potential extractables when evaluating elastomeric components to inform a risk assessment.
Elastomeric Analysis to Investigate Levels of Analytes with Concentrations Correlated to ICH Q3D PDEa
Several procedural modifications were required as part of the method development throughout this study. Ensuring accurate element recovery and sufficient method sensitivity were found to be key challenges. Three different extraction solvents were used initially to account for potential solubility and recovery differences arising from hydrogen ion concentration. A highly acidic pH was needed for ideal recovery of some metal elements, such as Cu and Sn (28, 29). Element Hg required further modification of the extraction solvent to deliver the appropriate sensitivity through the addition of Au as a complexing agent (30). Correction of spectral interference was also required for As through the forced addition of oxygen in the ICP-MS instrument using the DRC mode once HCl was added to the extraction solvent. Both 40Ca35Cl and 40Ar35Cl can be detected at mass 75, the same mass as As (31). These interferences were resolved by using the DRC mode of the ELAN DRC II. Oxygen is introduced briefly into the reaction cell and the 75As reacts with 16O to generate 75As16O with a resulting mass of 91. The interfering 40Ca35Cl and 40Ar35Cl do not react with oxygen and remain at mass 75, shifting the target analyte away from the polyatomic interferences to allow interference-free analysis of As. Other steps to maximize recovery in the method were also used, such as an exaggerated sample to solvent ratio beyond that of typical product conditions and the extraction of elements in a microwave oven at an elevated temperature.
As a result of this study, an elemental extraction and analysis method was identified that is capable of detection of a range of elements in an elastomer with a sensitivity of <0.05 μg/g as extrapolated from recommendations in ICH Q3D. The results show that elements of concern and, surprisingly, elements that are intentionally added in high amounts are recovered at low levels, even under extreme conditions. Further, the method development highlights that variations in solvents and element recovery should be considered when developing test methods to characterize elastomeric components. Elements are typically not water-soluble, difficult to extract, and in turn, difficult to predict, and elements that are commonly added to elastomers may be variable between individual components. Any other unexpected elements that are detected during risk assessment should be considered for potential quality concerns case by case. Elastomers are also only one potential packaging component that could contribute to an elemental impurity profile. Although the focus of this study was on rubber components, risk to drug product will need to evaluate potential accumulation from the entire packaging system; cases of elemental impurities have occurred from glass and plastic materials as well (11, 12, 22).
Conclusions
This study shows proof of concept that quantitative elemental impurity data can be generated from elastomeric closures to support the development of risk assessment and control strategies and also provides methodology as a starting point to generate these data. Further, low recovery of specified elements under exaggerated conditions highlights that elastomer contribution to an impurity profile could potentially be minimal. Although the extraction conditions are not typical of drug product-stopper contact conditions, the goal of the study was to identify elemental impurities detected with exaggerated, worst-case extraction conditions rather than to identify potential leachables. While exaggerated extraction conditions can facilitate understanding of any unanticipated chemical entities observed with analysis of the final drug product, chemical entities detected with aggressive extraction conditions are not representative of leachables detected under conditions that necessarily consider drug product storage conditions and duration, extraction propensity of drug formulation, and drug product/packaging component contact area. Once a full elemental characterization of specific packaging components has been achieved, the extractable elements can be narrowed to specific targets that are of higher toxicological concern or have a higher likelihood of occurrence. The most meaningful information can then be obtained through scientific evaluation of the drug product and its closure system together under standard storage conditions to determine the likelihood of elemental leaching resulting from drug product–elastomer interactions.
Conflict of Interest Declaration
All authors were employed by West Pharmaceutical Services, Inc. during execution of the research and/or generation of the manuscript.
- © PDA, Inc. 2019