Abstract
International Organization for Standardization (ISO) 15883 for washer-disinfectors has introduced the A0 concept to allow comparison of the lethality of moist heat processes. The A0 value is the equivalent disinfection time in seconds at 80 °C calculated on the basis of microbial killing kinetics when the disinfection temperature is over 65 °C. Hepatitis B virus (HBV), transmissible only to humans and chimpanzees, is an important heat-resistant, blood-borne pathogen. Therefore, it is mandatory to disinfect HBV thoroughly in the washer-disinfectors employed for surgical instruments. Additionally, it has become extremely difficult to use chimpanzees as experimental models or to perform human volunteer studies. Therefore, it is considered worthwhile to re-evaluate the reported data on the moist heat disinfection of HBV using the A0 value. In the voluntary active immunization to humans in 1973, HBV serum (infectivity titer: 106.5 CID50/mL) underwent moist heat disinfection at 98 °C for 1 min in a flask over an electric burner (conservatively estimated A0 value: 3786). Then, 0.1 mL was inoculated to each of 29 volunteers. No one revealed evidence of infection clinically or in the laboratory tests available at the time. In 1979, a more sensitive test appeared and revealed three sub-clinically infected volunteers. In the 1980s, there were two chimpanzee experimental models using HBV serum (infectivity titer: 105 CID50/mL). In one model, the serum underwent moist heat disinfection at 98 °C for 2 min in a thermostat bath (conservatively estimated A0 value: 7571). One milliliter was inoculated to each of two chimpanzees, and both of them revealed no evidence of infection. In another model, the serum underwent moist heat disinfection using two conditions in a thermostat bath, respectively: at 103 °C for 90 s (A0 value: 24865) and at 65 °C for 10 h (A0 value: 1138). Ten milliliters of each sample were mixed. Then, the mixture was inoculated to each of two chimpanzees. Both of them revealed no evidence of infection. In the human volunteer study, the serum infectivity titer was more than 30 times (101.5 times) higher than that used in the two chimpanzee experimental models. Moreover, the serum was heated in the flask over an electric burner, which is considered less reliable than the thermostat baths to realize uniform heat distribution. It is assumed that these factors were predisposed to the result that a conservatively estimated A0 value of 3786 failed to inactivate the HBV serum of 106.5 CID50/mL. In the two chimpanzee models, it was suggested that A0 value not less than 1138 was able to inactivate the HBV serum of 105 CID50/mL.
- Thermal disinfection
- Moist heat disinfection
- A0 value
- A0 concept
- Washer-disinfector
- Hepatitis B virus (HBV)
Introduction
Moist heat disinfection is performed in the washer-disinfectors employed for heat-tolerant surgical instruments. International Organization for Standardization (ISO) 15883 describes two methods for defining moist heat disinfection levels: a new concept of A0 value and a conventional method of time and temperature cycles (1–3).
A0 value is the equivalent disinfection time in seconds at 80 °C calculated theoretically on the basis of microbial killing kinetics. A0 value, which is a single parameter, allows easy comparison on the lethality of various time and temperature cycles. ISO 15883 defines that A0 value is calculated when the disinfection temperature is over 65 °C. This is because the parameters of microbial killing kinetics change dramatically below 65 °C in the thermophilic microorganisms, such as Hepatitis B virus (HBV) (1).
HBV, transmissible only to chimpanzees and humans, is important as a heat-resistant, blood-borne pathogen because it is a cause of chronic hepatitis, liver cirrhosis, and primary liver cancer (4). Consequently, it is mandatory to disinfect HBV thoroughly when surgical instruments are reprocessed in the washer-disinfector.
Additionally, it has become extremely difficult to use chimpanzees as experimental models or to perform human volunteer studies (5). As a result, it is considered important to study the reported data on the moist heat disinfection of HBV by using A0 value so that the optimal A0 value is clarified to inactivate HBV without the need for animal-specific testing.
Incidentally, Rosenberg reports on the optimal A0 value for inactivating HBV (6). However, in this report, the A0 concept was extrapolated to the plasma pasteurization at 60 °C for 10 h whereas ISO 15883 defines A0 value in moist heat over 65 °C (1, 6). A0 value is calculated in the temperature range above 65 °C in the microbial inactivation of products, which are able to withstand temperatures greater than 65 °C (1, 6). The plasma study may have been conducted at 60 °C so that thermal procedures would not affect the product adversely. Thus, it requires a long exposure time such as 10 h (6).
Therefore, in the present study, the reported data were studied by using A0 value, in which the disinfection temperature was over 65 °C. The study aimed to evaluate the optimal A0 value in the moist heat disinfection of HBV according to ISO 15883.
Materials and Methods
A0 Value
A0 value indicates the comparative lethality of moist heat disinfection (1). It is the equivalent disinfection time in seconds at 80 °C, when time and temperature cycles of moist heat disinfection are converted theoretically to the thermal processes at 80 °C. Theoretical conversions are performed on the basis of microbial killing kinetics.
Mathematically, A0 is expressed as
 where T is the measured temperature of the load in °C, and Δt is the selected time period in seconds. The Z value is constant at 10 °C in the A0 value (1).
where T is the measured temperature of the load in °C, and Δt is the selected time period in seconds. The Z value is constant at 10 °C in the A0 value (1).
The Z value indicates the required temperature to change the D value by a factor of 10, when the D value is the required time for a one-logarithmic reduction of microbial count. The A0 value is calculated when the temperature is over 65 °C. This is because the Z value may change greatly at the lower temperatures, and a number of organisms will proliferate actively below 55 °C (1).
Some microbes are reported to be susceptible to the moist heat at approximately 50 °C: Cryptosporidium oocysts, human immunodeficiency virus, Streptococcus, Salmonella, Candida, Aspergillus spores, and so forth. Legionella, Enterococcus, Coxiella, Mycobacterium species, parvoviruses, noroviruses, and HBV are examples of microbes that survive the moist heat above 45 to 50 °C. The spores of Bacillus, Geobacillus, and Clostridium are considered most resistant to moist heat. A temperature over 100 °C is used to inactivate the bacterial spores in the autoclave (7, 8).
A0 value consists of the lethality in the come-up, holding time, and come-down phases. The come-up phase starts at 65 °C and ends when the programmed disinfection temperature is attained. The holding time phase is the period in which the disinfection temperature remains above the programmed value. Then, the come-down phase follows and ends when the disinfection temperature decreases to 65 °C (1).
Search Strategy for Reported Data on the Moist Heat Disinfection of HBV
Reported data on the moist heat disinfection of HBV were identified using the following methods. Electronic searches of MEDLINE from 1950 to March 2009 were performed. The search terms included HBV, heat, thermal disinfection, moist heat disinfection, pasteurization, and chimpanzee. Additionally, the bibliographies of all publications were reviewed, and related bibliographies were manually searched for relevant references that might have been missed in the database search.
Then, the reported data on either human volunteers or chimpanzees were selected that satisfied the following two conditions. One, that the infectivity titer was known in the employed HBV serum. The other, that the disinfection temperature was over 65 °C. In addition, the presence of informed consents and conformity to the appropriate ethical codes was confirmed in the human volunteers study.
In each of the identified reports, the following data were collected: year of publication; strain, subtype, and infectivity titer of initial HBV serum; methods to dilute initial HBV serum; materials employed to dilute initial HBV serum; volume and infectivity titer of the samples to be heat-inactivated; methods to heat and cool the sample; thermometric monitoring methods; thermal data in the come-up, holding time, and come-down phases; dose and route for inoculation; details on the inoculated subjects; follow-up periods after inoculation; diagnostic methods used to detect HBV infection; and the results of the diagnostic methods. As to the subtypes of HBV, four major serotypes are known. They are designated adw, ayw, adr, and ayr (9).
Estimation of A0 Values
A0 values were estimated according to the A0 value definition in ISO 15883 using the reported data (1).
In each of the reported data, A0 value was calculated in the come-up, holding time, and come-down phases, whenever sufficient thermometric data were available. Then, these calculated A0 values were summed up to estimate A0 value. When thermometric data were recorded only in the form of a graph, the data were read from it.
Results
To the best of the authors' knowledge, there were eight reports considered eligible for evaluating the moist heat disinfection of HBV. Five of them employed the pasteurization of HBV at 60 °C for 10 h. The three remaining reports employed disinfection temperatures not less than 65 °C (Table I): one case of voluntary active immunization to humans in the 1970s and two chimpanzee experimental models in the 1980s (10–22).
Moist Heat Disinfection of HBV
Voluntary Active Immunization to Humans in 1979 (Table I)
The MS-2 strain of HBV serum (107.5 CID50 units/mL) was used (15–19) (CID50: a dose that infects 50% of chimpanzees). Children participated in the study only after documentation of the parents' consent, approval by local, state, and federal agencies, and compliance with the World Medical Associations Draft Code of Ethics on Human Experimentation from 1961.
MS-2 was diluted using sterile distilled water. The diluted serum (106.5 CID50/mL) underwent moist heat disinfection in a 50 mL flask over an electric burner: 43 s of heat-up time, maintained at 98 °C for 1 min, and cooled to room temperature in 25 min. The temperature was monitored using a thermometer. Not using come-up and cool-down heat temperatures in the calculation provides a conservative estimate of the A0 value of 3768 that was used for this study.
The heat-treated dilution of 0.1 mL was injected intramuscularly to each of 29 susceptible volunteers. They were followed up for eight and half months clinically and by using laboratory tests. Complement fixation or reversed passive hamagglutination was used to detect HBsAg (HBV surface antigen). All volunteers revealed no evidence of infection in 1973.
In 1979, a more sensitive radioimmunoassay method (Ausria II) appeared and detected HBsAg in the three volunteers. Krugman and coworkers described in their report that the three volunteers showed a markedly attenuated sub-clinical infection without any evidence of liver involvement (19).
Chimpanzee Experimental Model in 1984 (Table I)
An HBV serum of JHB001 strain (108 CID50/mL) was diluted using a phosphate buffer (pH 7.2) (20, 21). One milliliter of the diluted serum (105 CID50/mL) was heated in the thermostat bath for 6 min. Preliminary thermometry using a thermocouple revealed 4 min of heat-up time and 2 min maintained at 98 °C. The sample was cooled rapidly in an ice water bath. Not using come-up and cool-down heat temperatures in the calculation provides a conservative estimate of the A0 value of 7571 that was used for this study.
The heat-treated dilution of 1 mL was administered intravenously to each of two susceptible chimpanzees. They were followed up for 30 weeks using laboratory tests and liver biopsies. Both of the two chimpanzees revealed no evidence of infection.
Chimpanzee Experimental Model in 1987 (Table I)
Three intermediate products of plasma-derived HBV vaccine were prepared under the same circumstances to manufacture the vaccine: inoculums I, II, and III, respectively. Inoculum I was the ultracentrifuge resuspension in PBS (phosphate buffer saline), and its HBV infectivity was eliminated after the chemical purification step (22).
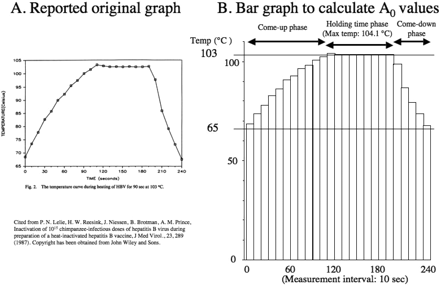
Inoculum I was spiked with the HBV serum at 107 CID50/mL (NYBC 78-564) so that the final titer would be 105 CID50/mL. This HBV-spiked solution underwent moist heat disinfection at 103 °C for 90 s, heated and cooled in the thermostat bath. This heat-inactivated solution was ultracentrifuged and sterile-filtered to produce inoculum II. The temperature was continuously monitored using a calibrated data-logging apparatus (Kaye Instruments) every 10 s. Thermometric data were available only in the form of a graph (Figure 1A). The A0 value was 24,865 (Figure 1B, Table II) (22).
(A) Time versus temperature graph cited from reference 22. (B) Figure 1A converted to the bar graph. Each bar has a width of 10 seconds, which was a measuring interval in the thermometry. The height of each bar represents the actual temperature. In each bar, an equivalent disinfection time at 80 °C was calculated on the basis of microbial killing kinetics. The obtained equivalent time values were summed up to the estimate A0 value, when the actual temperature was over 65 °C (Table II).
Calculation of A0 Value in Moist Heat Disinfection at 103.0 °C for 90 s
The HBV-spiked protein solution (105 CID50/mL) underwent moist heat disinfection at 65 °C for 10 h in the thermostat bath (A0 value: 1138) to produce inoculum III. It was a dilute solution prepared using PBS and containing 1 mg/mL of protein. The temperature was continuously monitored using a calibrated data-logging apparatus (22).
The mixture of inoculum II (10 mL) and inoculum III (10 mL) was injected into each of two susceptible chimpanzees. They were followed up for 28 weeks using laboratory tests and liver biopsies. Both of the two chimpanzees revealed no evidence of infection (22).
Discussion
HBV is an important heat-resistant pathogen in the prevention of healthcare-associated infections. However, HBV is transmissible only to chimpanzees and humans (4, 5). For this reason, the reported data on the moist heat disinfection of HBV are considered a valuable source of information.
ISO 15883—Washer-Disinfectors has been approved recently and introduced a new concept of A0 value to indicate moist heat disinfection levels (1–3). Accordingly, it is considered worthwhile and timely to study the reported data on the moist heat disinfection of HBV by using A0 value.
Bibliographically, there were three reports on the moist heat disinfection of HBV suitable for the present study. There was one report on the voluntary active immunization to humans reported in the 1970s and two reports on chimpanzee experimental models reported in the 1980s (15–22).
The lethality of moist heat disinfection could be affected by several factors besides A0 value. They include the sensitivities of diagnostic methods to detect HBV infection, infectivity titers of HBV prior to disinfection, heat distribution in the thermal process, suspension media of HBV, and so on (7).
In the human volunteer study, a more sensitive diagnostic method appeared in 1979 and revealed three sub-clinically infected volunteers. The infectivity titer of the HBV serum sample in this study was 106.5 CID50/mL, which was more than 30 times (101.5 times) higher than that employed in the two chimpanzee experimental models (15–19).
Moreover, in this human volunteer study, the HBV serum sample was heated in a flask over an electric burner. This heating method is considered less reliable to realize uniform heat distribution than the thermostat baths employed in the two chimpanzee experimental models. It is assumed that these factors predisposed to the result that conservatively estimated A0 value of 3786 failed to inactivate the HBV serum of 106.5 CID50/mL completely (15–19).
In both of the two chimpanzee experimental models, the infectivity titer of HBV serum samples was identical at 105.0 CID50/mL, and these HBV serum samples were heated in thermostat baths. Three heating conditions were tested in these two chimpanzee models: 98 °C for 2 min (conservatively estimated A0 value of 7571), 103 °C for 90 s (A0 value of 24865), and 65 °C for 10 h (A0 value of 1138) (1, 14). All of these three conditions inactivated HBV successfully. Therefore, it was suggested that A0 value not less than 1138 was able to inactivate the HBV serum of 105.0 CID50/mL (20–22).
The World Forum for Hospital Sterile Supply (WFHSS) recommends an A0 value of 3000 to inactivate HBV on the basis of ISO 15883 (23). Practically, the maximum HBV infectivity titer is speculated at 108 CID50/mL in human body fluids (24). When a cleaning process achieves a three-logarithmic reduction in infectivity, the HBV titer would be 105 units/mL at most after cleaning (25). Hence, it might be considered that WFHSS recommends twice as much as the A0 value required to inactivate HBV with a sufficient safety margin.
Incidentally, Bliem and Nowak state that the feasibility of a constant Z value at 10 °C should be studied when A0 value is applied, whereas it is internationally approved in ISO 15883 (26, 27). However, susceptible hosts of HBV are too limited to afford the sufficient thermal kinetics of HBV (28). Pflug states that a Z value should be assumed until a more accurate Z value is revealed (29).
Under these circumstances, surrogate viruses for HBV have been pursued in the experimental models (30, 31). For instance, bovine parvovirus (Haden strain, BPV) is recommended as a surrogate for HBV in the moist heat disinfection. BPV is handled easily using sub-cultured fetal calf lung cells (28). Thermal kinetics of BPV have been studied in the moist heat from 75 to 90 °C, when BPV was suspended in distilled water, plasma, and water of standardized hardness (WSH) (32).
At 80 °C, D values of BPV were 7.5 min in the distilled water and 23.6 min in the WSH. In plasma, BPV showed a biphasic pattern and yielded D values of 1.5 and 17.9 min. It is suggested that BPV was most resistant in WSH and least resistant in the distilled water. The Z values were 5.6 °C in the distilled water and 8.2 °C in the WSH (32).
In the human volunteer study, the HBV serum MS2 was diluted using distilled water by 1:10 (15–19). In the chimpanzee model of Kobayashi and coworkers, the HBV serum JHB001 was diluted using phosphate buffer by 1:1000 (20, 21). In the chimpanzee model of Lelie and coworkers, inoculums II and III were prepared on the basis of the ultracentrifuge resuspension of PBS. Protein concentration was reported only in the inoculum III as 1 mg/mL (22).
It is assumed that the suspension media in the chimpanzee models and human volunteer study were comparatively less similar to either the plasma or distilled water in the BPV study (33, 34). It is reported that the Z value of BPV in WSH was 8.2 °C (32). Therefore, from a practical viewpoint, it is considered possible to apply A0 concept (Z value at 10 °C) to the data on the inactivation of HBV reported in the human volunteer study as well as the two chimpanzee experimental models (35).
The Centers for Disease Control and Prevention suggests that surrogate viruses for HBV, such as Duck Hepatitis B Virus (DHBV), should play an important role (36). For this purpose, it is considered helpful to re-evaluate using A0 concept the data on the moist heat disinfection of HBV available in the human volunteer study and chimpanzee experimental models. This effort is considered to pave the way for further studies using surrogate viruses of HBV.
Conclusions
Reported data on the moist heat disinfection of HBV in the human volunteer study and chimpanzee experimental models were re-evaluated using A0 value defined in ISO 15883 for washer-disinfectors. It was suggested that A0 value not less than 1138 was able to inactivate HBV serum with the infectivity titer of 105 CID50/mL.
- © PDA, Inc. 2010