Abstract
This article describes a method for achieving the load equivalence model, described in Parenteral Drug Association Technical Report 1, using a mass-based approach. The item and load bracketing approach allows for mixed equipment load size variation for operational flexibility along with decreased time to introduce new items to the operation. The article discusses the utilization of approximately 67 items/components (Table IV) identified for routine sterilization with varying quantities required weekly. The items were assessed for worst-case identification using four temperature-related criteria. The criteria were used to provide a data-based identification of worst-case items, and/or item equivalence, to carry forward into cycle validation using a variable load pattern. The mass approach to maximum load determination was used to bracket routine production use and allows for variable loading patterns. The result of the item mapping and load bracketing data is “a proven acceptable range” of sterilizing conditions including loading configuration and location. The application of these approaches, while initially more time/test-intensive than alternate approaches, provides a method of cycle validation with long-term benefit of ease of ongoing qualification, minimizing time and requirements for new equipment qualification for similar loads/use, and for rapid and rigorous assessment of new items for sterilization.
- Steam sterilization
- Variable load
- Mixed load
- Autoclave
- Validation
- Risk-based approach
- Load equivalence
- Load bracketing
- Item bracketing
Statement of Objectives or Hypothesis
In an effort to minimize the frequent sterilization validation studies required for a multiproduct facility, where new product/component configurations requiring steam sterilization are routinely introduced, a method was needed to objectively determine the worst-case equipment/commodities for routine validation testing. The validation method chosen had to fulfill several key objectives:
-
Characterize and document all equipment and components requiring steam sterilization.
-
Identify the subset of equipment and the subset of components that comprise a worst-case load relative to sterilization.
-
Provide a mechanism to assess new components and equipment configurations and determine whether the item fits within the current qualification matrix or constitutes a new-worst case item.
-
Provide a standardized test methodology such that all newly identified worst-case equipment and components could be tested, with the results being directly comparable to previous qualification studies.
The general sterilization philosophy employed was the bracket approach, wherein both the maximum load size (based upon mass) and the minimum load size, for each unique load type, were evaluated and challenged to identify the worst-case load for performance qualification (PQ) testing. The item and load bracketing approach allows for load size and load pattern variation for operations flexibility.
Introduction
A process improvement program was initiated to enable the introduction of new product processing configurations to the production floor in a timely manner, while maintaining a high level of assurance that the new component introduction did not compromise the sterility of currently qualified components or risk contamination of the aseptic processing area.
Initial sterilization qualification studies had at one time been performed using a fixed loading pattern approach as a means to expedite the project schedule. While this approach expedited initial facility qualification and licensing, operational issues resulting from the inflexibility of having fixed loading patterns emerged soon after initiation of product manufacturing. Chief among the operational issues encountered were
-
As process changes were identified to optimize filtration and filling operations, seemingly constant requests were being generated to change the previously qualified loads.
-
Equipment/component preparation area operators, in an effort to accommodate production demands, were frequently requesting reevaluation of the loading pattern constraints, often on a load-by-load basis. For example, could a filter cartridge that is typically sterilized on the top shelf of a load located next to a coil of product tubing now be added to an existing load and sterilized using the identical cycle parameters but located on the third shelf of an autoclave cart next to a product filling manifold?
-
The fixed load patterns lent themselves to “common sense” interpretations, resulting in load patterns for which no qualification data had been acquired. For example, a filter cartridge that was qualified to be loaded on the top shelf, in the front right corner, is found loaded in the middle of the top shelf. Is this an issue, and if so, how much leeway does the operator have? Lacking definitive data on how much variation is allowable without affecting sterilization efficacy, this is a difficult if not impossible question to adequately answer and thus presents a significant obstacle to effective training of equipment preparation operators. Although reasonable and rational scientific thought supports the ability to relocate but not reorient objects, the fixed-load approach initially utilized did not provide sufficient data to identify process boundary conditions.
To address the issues observed, a new qualification approach was developed. The approach chosen applied the item bracketing and load bracketing concepts presented by Parenteral Drug Association (PDA) Technical Report 1, 2007 (1). The item bracketing approach allows for the use of worst-case items (i.e., greatest sterilization challenge for heat penetration, mass, air removal, and/or condensate removal) to qualify items that are a lesser sterilization challenge. The load bracketing is established by using predefined minimum and maximum loads. This approach defined the minimum load as comprising the single item presenting the greatest sterilization challenge, irrespective of mass, while the maximum load comprised an assembly of the 10 items identified as worst case (relative to sterilization difficulty), with additional items used to create a maximum load mass (i.e., a load mass greater than any anticipated for routine operations).
This approach requires that several key criteria are addressed during qualification and the subsequent process transfer to manufacturing:
-
The sterilization cycle parameters used for validation utilize the overkill approach to assure any variability in loading pattern or orientation is accounted for during routine production. (Note: While the basic precepts of this validation methodology can be applied to other non-overkill validation approaches [e.g., F0/bioburden based sterilization, sub-lethal bioburden reduction cycles, etc.], such approaches require additional bioburden monitoring, control, and evaluation components that were outside the scope of this case study.)
-
Given the requirement for an overkill approach, multiple maximum and minimum loads may be required, wherein heat-labile (e.g., elastomeric materials, closures, etc.) and nonheat-labile (e.g., stainless steel parts, etc.) are segregated into separate load types. Where all equipment and components can withstand sterilization cycle parameters sufficient to provide a sterility assurance level (SAL) of ≥106, such segregation may not be necessary.
-
The variability in loading pattern and orientation must be addressed through specific and detailed procedural controls because the process of loading porous load autoclaves for aseptic filling/manufacturing facilities is manual.
-
The documentation system must clearly define the items that were qualified with a maximum quantity that can be loaded, and it must provide the ability to fully document the specific load contents to allow verification of process compliance during batch record review.
-
The system must allow for the contents of individual loads to vary, while maintaining consistent sterilization cycle parameters and performance, ensuring a consistent level of sterility assurance.
Heat penetration mapping studies should be conducted on all items to identify the more difficult-to-heat items (e.g., large mass, potential for trapped air or condensate collection, hoses with longest lengths, or any combination of these issues). When conducting item temperature mapping, it is important to consider the types of challenges the item may represent (e.g., air removal versus significant mass) and to position the temperature probes in slowest-to-heat locations (2). For larger load items, items of complex construction, or items where mass distribution is highly nonuniform, multiple probes may be required to ensure that the coolest location on/within each item is clearly defined. In some instances (e.g., large filter housing/valve/tubing assemblies, large bags of closures, intermediate tanks, etc.), separate item-mapping studies may be indicated as a precursor to load heat penetration studies. Such item-mapping studies minimize the monitoring requirements for large or complex items during subsequent load heat penetration tests, freeing up probes for monitoring of other load items, and thus decreasing the total number of heat penetration test cycles required.
The methods described in this article result in a sterilization and documentation control system that
-
Has a controlled version of a batch preparation list. This list is attached to preparation procedures where items are not separated out by specific load but are listed individually. Each autoclave load configuration is documented on the batch preparation list form during routine operation.
-
The validation will be run using a minimum load and a maximum load to validate combinations of different items to show consistency among configurations. The test method will demonstrate that while it is recognized that the preparation and orientation of each load item is critical, the item's location within the load is not critical.
-
The maximum loads studies will be conducted with a load containing a total mass in excess of that needed for routine manufacturing. This maximum mass will establish quantities of items that may be processed in a single load.
-
Based on the validation, a master list of maximum items in the load will be established.
Method and Materials
The methods used for this qualification comprised the following primary activities:
-
Listing of all equipment/commodities to be steam sterilized.
-
Evaluation of equipment and commodities to determine the most efficient and effective means (relative to air removal, steam penetration, and maintaining a sterile barrier) of pre-sterilization preparation.
-
Heat penetration evaluation tests to identify the worst-case (i.e., most difficult-to-heat) load items.
-
Maximum load (i.e., greatest mass) heat penetration/biological challenge studies using the worst-case load items.
-
Minimum load (irrespective of mass) heat penetration/biological challenge studies using the single worst-case load item.
-
Evaluation of data and subsequent generation of loading, operation, and documentation procedures for manufacturing use.
A listing of all commodity items requiring steam sterilization was developed using existing procedures and batch record documentation. The component preparation instruction for each of the listed commodities was reviewed for clarity and consistency, and preparation methods were standardized wherever possible.
The initial worst-case item heat penetration evaluation studies were performed for each unique load type (e.g., stoppers, seals, filling equipment, sanitization supplies, etc.), and/or for each unique cycle type (i.e., vacuum pre-purge, gravity displacement, etc.). While multiple autoclaves were used for this study, only one autoclave was used for any one of the unique load types evaluated in order to ensure comparability between test cycles (e.g., stopper studies in a single unit, equipment studies in a single unit, etc.). Because the purpose of this test was to determine the relative heat-up characteristics of the equipment and component items within each load, testing of all load types in each autoclave was not required for determination of the worst-case commodities.
Commodities were prepared by rinsing with water for injection (WFI), then smaller items (less than two square feet in area) were bagged and sealed in steam-permeable bags, while larger items had all open ends/ports covered using steam-permeable, draw-tight bonnets (General Econopak 1030 series mini-muff). Each commodity was weighed after preparation and prior to loading to record the mass of each load. The mass approach to individual items and the maximum load size was used in recognition that commodity heating, and the generation of condensate in the load, is primarily mass-dependent. The efficiency with which the condensate is removed by the sterilizer is critical for adequate steam penetration of the load items (3).
The orientation of each commodity was documented to provide loading procedures to the operators during PQ testing and future routine operations. Items were oriented, where practicable, to minimize the possibility of condensate collection, and maximize air removal efficiency. Calibrated thermocouples were placed within each commodity to measure the temperatures achieved during the cycle and to calculate a lethality value (F0 calculated using a D-value of 1.0, z-value of 10 °C, and reference temperature of 121.1 °C) achieved during the entire cycle for each commodity. (For this case study, 121.1 °C was chosen as the baseline sterilization temperature, although it should be noted that other baseline temperatures may be employed to address specific component requirements and/or validation strategies.) Care was taken to seal the wrapping material around the thermocouple penetration to prevent enhancement or restriction of steam penetration into the item.
Following the evaluation runs of each commodity, the data were analyzed to determine the two worst-case commodities within that load based on the following parameters: Longest lag time from exposure start to attaining ≥121.1 °C, shortest dwell period at ≥121.1 °C, lowest maximum temperature attained during the exposure (dwell) period, and lowest accumulated lethality (F0). These two worst-case items were then allowed to cool to ambient conditions and placed in the next load along with untested commodities for further comparison. Inclusion of the two worst-case items into each subsequent load provided a facility for evaluating cycle-to-cycle variability that could affect the evaluation process (i.e., if the thermal profile was comparable for these two items, cycle to cycle, then thermal variability within the remaining load items can be assumed to be component-related, not cycle-related). This process was repeated until all commodities identified for analysis were tested at least once. The data from all runs was then compared to identify the 10 worst-case commodities overall that would be used for PQ testing of each sterilizer, using identical bagging/wrapping methods.
Following the identification of the 10 worst-case commodities, the minimum and maximum worst-case loads (of each unique load type) were assembled and processed for three consecutive successful PQ runs to verify that the load preparation, load configuration, and sterilizer parameters were satisfactory for routine operation. For each of the three PQ runs the commodities were moved within the load to verify that the commodity was successfully sterilized regardless of its position in the load and its nearest neighbor commodity. Each load item was tested, as near as practicable, in the front, middle, and back of the chamber and in the bottom, middle, and top of the chamber (e.g., bottom-front, middle-middle, and top-back).
Results
The initial assessment of components requiring sterilization was completed through operator interviews and batch record reviews. It was critical to identify the commodities required to be steam sterilized and to document the preparation methods used for sterilization of those items. For the method of variable load validation to achieve consistent results it is important to control the commodity preparation, including pre-rinsing and bagging/wrapping, along with the commodity orientation during sterilization.
Various commodity orientations were tested during the initial development studies to verify that either the orientation was not critical to the sterilization of an individual item, or that the item orientation is critical for complete draining and efficient steam penetration/air removal. Areas of steam penetration concern were selected for thermocouple monitoring. These included areas such as domed sections with nonsymmetrical openings that may trap air, in the center of tubing lengths, and in areas of greatest mass concentration of the individual commodity. All commodities used in the challenge tests were prepared (wrapped, bagged, wet, dry, etc.) in the same manner as for manufacturing use. Each commodity was loaded in the same physical orientation (i.e., horizontal, vertical, inverted, etc.) during each of the qualification runs, while the location of the item was varied from front to back and top to bottom.
Thermal equivalence between loads of varying load configurations and mass was evaluated by comparing the penetration thermocouple data obtained from the various loads, when processed using identical sterilization parameters, in a single autoclave (Table I). The heat penetration data demonstrated that the thermal profile for each of the items tested was equivalent at each tested location (i.e., front, middle, back; top, middle, bottom of chamber), verifying that the load “cool spot” was load item-dependent, not load position-dependent. The thermal profile data were also evaluated to identify the 10 worst-case items, relative to all other items planned for steam sterilization (Table II). This result was verified by repositioning the load items during the replicate runs and verifying that the worst-case item remained the worst case relative to the other load items. The data presented in Table II detail the number of times each individual item was identified as the worst-case item, relative to the other load items, for each of the specific assessment criteria. It is clear from the data in the table that two filters, each with a long section of tubing with a valve on one end, were the overall worst-case items based on the total number of times they were observed within the top 10 coolest items in a load. The other items identified in the top 10 varied in their ranking depending on the criteria used for comparison. For example, the cleaning hose did not have a lag time at the beginning of the exposure period, indicating quick heat-up characteristics, but also did not achieve a temperature as close to the load set-point, relative to other items, resulting in a lower accumulated lethality. The hose was also quick to cool down, resulting in a shorter total time above 121.1 °C relative to other items, and this therefore also contributed to the lower accumulated lethality for this item. Comparing the hose results with those of the stoppering outer bowl demonstrates that the more massive outer bowl took longer than the hose to achieve the sterilization temperature of 121.1 °C but was able to achieve a higher maximum temperature and higher accumulated lethality. The observation that the worst-case items maintained a constant thermal profile and that the profile was consistent relative to the thermal profiles of the other items in the load, irrespective of load configuration or item location, provides the basis for elimination of fixed loading patterns in routine sterilization. The items in the shaded rows of Table II were within the top ranking for worst-case items within a single test run but did not consistently rank in the top 10 relative to the other items tested. Although the 25-foot section of silicone tubing did not rank in the top 10 worst-case items, it was selected for PQ testing because the results were very similar to the worst-case items ranked 8 through 10, two of which were the same item prepared in different ways. Based on the cool point determination study data, four PQ runs (3 maximum loads and 1 minimum load) were executed to validate the process. Since the minimum and maximum loads are determined to be equivalent relative to delivery of a ≥106 SAL, loads of intermediate size are deemed equivalent as well.
Cycle Sterilization Parameters
Cool Point Determination for Commodities
The thermal data from the cool point determination study identified the 10 worst-case items to be challenged during PQ execution. The worst-case items along with additional items (dummy items for mass) were assembled to achieve a total load mass that exceeded that anticipated for routine production (Table III). Cool point determination studies for the minimum load were not required due to the small volume of materials loaded, the relatively small area occupied by the load contents, and the high percentage of the load being monitored (i.e., ≥50%). Therefore, four PQ runs (3 maximum loads at 175 kg and 1 minimum load at 0.5 kg) were executed to validate the process. The item identified in the maximum load studies as the hardest-to-heat item was used for the minimum load qualification.
Proposed PQ Challenge for Filling Equipment Maximum Load
Overall Equipment Listing
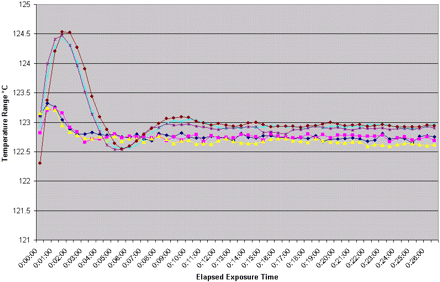
During the maximum load tests, all penetration thermocouples accumulated ≥15 min F0. The minimum F0 was 33 min for run 2B as shown by the minimum temperature for that specific run (Figure 1). There were no criteria during PQ testing to maintain a minimum temperature or lag time after the initiation of the exposure period. The cycle exposure period was started based on distribution temperatures within the chamber and chamber drain remaining above 121.1 °C and within a 1.5 °C spread. It is important to note that efforts (e.g., equipment disassembly, cycle development) should be made to minimize any lag time in accordance with the EU expectations (4). However, the methods employed to meet this lag time requirement should be carefully evaluated to ensure that the overall sterility assurance of the process is not adversely affected (e.g., by requiring an inappropriate number of aseptic connections) (5).
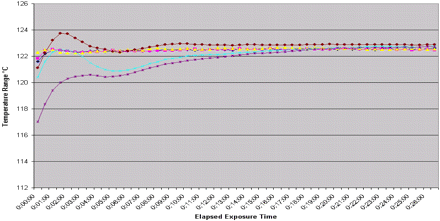
Fill equipment − max load minimum temperatures − from all penetration TC's > 121.1 °C until exposure cycle end.
In all runs the distribution temperature spread was less than 1 °C within the first minute after starting the exposure period (Figure 3). This assured an even temperature distribution within the chamber to provide equal exposure of the loaded items to steam during the cycle. The equal exposure of items within the chamber provides support for the ability to load any item at any shelf position within the chamber and not influence the sterilization capability.
Discussion
For the method of variable load validation to achieve consistent results it is important to control the commodity preparation, including pre-rinsing and bagging/wrapping, along with the commodity orientation during sterilization. Control of these items is necessary to minimize equilibration times of the commodities. Extended equilibration times, in addition to resulting simply from heterogeneous load contents, may also be indicative of inadequate air removal or steam penetration and, as such, should be evaluated and addressed (through cycle parameter modifications, preparation procedure modifications, etc.) where indicated to ensure that variability is minimized. This is of special concern during developmental and cool point mapping/penetration studies relying solely on thermometric data, where dry heat cannot be distinguished from moist heat. While there is an understandable desire to create assemblies of items for sterilization, to reduce the number of aseptic connections required in the aseptic processing area, care must be taken to ensure that the preassembled sections do not create excessive difficulties in air removal and steam penetration. In order to reduce or eliminate equilibration times, especially when complex wrapped/bagged items are being processed, care should be taken to orient items for efficient air removal (e.g., hoses not pinched, maximizing surface area for steam penetration, inversion of items with large internal volumes, etc.), increase the number of vacuum or positive steam pulses, add hold steps during vacuum and/or steam pulses, increase depth of vacuum pulses, and/or optimize steam exposure to load items by creating reasonable assemblies. In this qualification, non-wetting bags/covers were used for equipment preparation. However, in the case where wetable sterilization wraps are utilized, it is important to recognize that the first vacuum air removal pulse will be the most efficient (as the wrap becomes hydrated and thus less steam-permeable) following the first steam backfill. In this case, both the depth and duration of the first vacuum pulse should be maximized.
Initial development studies verified that while the physical orientation of an individual load item may be critical (for air removal and steam penetration), its location within the chamber did not affect its thermal profile. The data presented in Table II demonstrates the need for multiple assessment criteria to determine item and load equivalence, especially to provide sufficient definition of item performance on a cycle-by-cycle basis, to evaluate whether observed variability was cycle-specific or load item/location-specific. Because this was an inter-item comparative study using identical sterilization cycle parameters and similar preparation procedures, it was observed that for some items the lag time (to ≥121.1 °C) did not correlate with delivered lethality. For example, item A has a shorter lag time than item B but may achieve a lower overall mean kinetic temperature and therefore a lower lethality (assuming comparable steam penetration levels) than item B. This disparity in the ability to equilibrate quickly during exposure can be due to a number of factors, such as the specific heat of the various items based on their materials of construction, density and thermal conductivity, air removal efficiency, and localized superheat conditions (e.g., large hydrophilic absorbent pack in a breather bag). Thus two items of equal mass loaded into the sterilizer may have dissimilar thermal profiles where, for example, one is made of dense, high heat capacity and highly heat-conductive material, such as stainless steel, while the other is made of a lower density, low specific heat, and lower heat-conductive material such as polycarbonate. Thus, while it takes more energy to heat 1 kg of an item with a higher specific heat than an item of comparable mass with a lower specific heat, the lower specific heat/lower heat conduction item may take longer to heat. Additionally, while the maximum steam input to the autoclave chamber (e.g., during initial heatup) is constant, an item that has a very high specific heat and conductance will condense steam rapidly compared to other low specific heat and low heat conductance load items. As the steam collapses around the high mass/specific heat item, the localized low pressure acts as a “pump” to backfill the area with steam. Thus while the autoclave controls are based (typically) on one temperature monitoring point, the heat input may vary dramatically on a localized basis. A similar situation may be encountered when highly absorbent materials are sterilized, resulting in localized superheat conditions until the materials quickly reach an equilibrium hydration point. In the case of highly absorbent materials (e.g., wipes, batch record materials, etc.), evaluation of just a single comparison criterion (e.g., minimum temperature) may fail to detect item issues such as localized superheat that an evaluation of heat-up times and maximum temperatures would achieve or that an evaluation of minimum temperatures and F0 might identify. These issues highlight the criticality of using multiple evaluation criteria when determining worst-case components within highly mixed loads. This process verifies that the identified “cold spots” move in the chamber with the worst-case item (6). The final assessment of an item will be performed using a biological indicator to verify that the desired steam penetration has been achieved because “the bugs don't lie” (7). It should also be noted that while this study used only the lethality accumulated during the stabilized dwell period for evaluation purposes, approaches incorporating lethality accumulated during heat-up and cool-down can be equally valid, especially for heat-labile components for which exposure should be minimized. In the context of an overkill approach for non–heat-labile components, such as this case study, ignoring heat-up and cool-down lethality contributions simply increases overkill. Furthermore, the requirement that a certain minimum temperature be maintained during the exposure period (i.e., not less than 121 °C) for the cycle to be acceptable is not critical to the process as it relates to lethality or sterility assurance. The maintenance of moist heat conditions appropriate for sterilization, along with total cycle lethality (as calculated thermometrically), should be considered the critical minimum criteria for cycle acceptance, not simply minimum temperatures achieved.
Equivalence between loads of varying size for the same size chamber can be established by comparing the penetration thermocouple F0 range obtained from minimum and maximum loads using the same sterilization parameters in an autoclave. Data from the distribution thermocouple graph (Figures 2, 3) demonstrate that all areas within the chamber are equally exposed to steam. The penetration thermocouple data graph (Figure 1) demonstrates equivalent steam penetration into all loaded commodities for all runs. This demonstrates equivalence in all runs where the commodity locations where changed. If the data demonstrate equivalence, a total of four runs (3 maximum loads and 1 minimum load) can be used to validate the process (9). If the minimum and maximum loads are determined to be equivalent, then loads of intermediate size can be deemed equivalent as well.
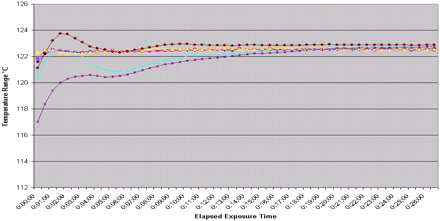
Fill equipment − max load minimum temperatures − from all distribution TC's > 121.1 °C until exposure cycle end.
Fill equipment − maximum load − temperature range of all distribution TC's > 121.1 °C to exposure cycle end.
The similarity of the distribution data for runs within the same autoclave (Figure 2) coupled with the pattern of penetration thermocouple data demonstrates the consistency of the assessment method within an autoclave. The confirmation of this consistency was established through the repositioning of load items in each of the replicate runs. The item repositioning data obtained during PQ supports the ability to eliminate fixed loading patterns in routine sterilization. This consistency of response of an item to the sterilization conditions provides the ability to assess an item in one autoclave and use that information to support that its response in another autoclave will be similar. Experimentation has shown that the item that is most difficult for steam to penetrate in the component mapping studies will most likely be the cold spot within the autoclave regardless of its location (8, 10). Therefore, the heating characteristics of any given item are dependent on that item's composition, preparation, and orientation and is independent of the autoclave used (assuming previously demonstrated equivalence between the autoclaves).
Once the validation has been completed and the listing of worst-case items has been developed, a risk-based assessment method can be developed to assess new items to be sterilized. The assessment method should include a risk review (11) of the intended preparation process (rinsing and wrapping) relative to the established item process, configuration within the load, and similarity to other items previously validated. The risk assessment may be able to justify that no validation testing is required for new items to be sterilized based on their equivalence to previously validated items. For example, validation testing of a bag of 3000 stoppers may not be required if previous validation testing has been completed for equivalent-sized stopper bags of 2000 and 5000 stoppers that were filled with stoppers made of rubber compounds with equal or higher substrate-specific D-values.
The risk evaluation is accomplished by grouping items to be sterilized based on their shape, materials of construction, preparation procedures, and preassembly methods (12). The preparation and preassembly methods for new equipment or components may be modified to conform with those of previously qualified, similar items to mitigate or reduce risks relative to both sterilization efficacy and maintenance of sterility during subsequent aseptic assembly and/or use procedures. If the risk assessment results in an item being classified as requiring additional evaluation (temperature mapping in a cycle including existing worst-case items) because it appears dissimilar to previously qualified items, then the original assessment criteria and worst-case items data from Table II will be used to perform a sterilization study to directly compare the new item to existing item sterilization data to determine if the new item is indeed a new worst case. Identification of a new worst-case item will require requalification of the sterilization cycle with the new items.
Review of the data presented here indicates a strong correlation between the thermometric properties of an item and the achieved lethality (as verified by bioindicator challenges) after identifying the appropriate commodity preparation methods and loading orientation. Based on the observed correlation, using only the thermometrically calculated item lethality in a comparison study of a new item to an existing item (in lieu of the four assessment criteria used in this study) may be justified. This approach appears feasible for items with similar configuration, preparation methods, and mass as previously qualified components. New items that are unique in configuration, and may present heat-up and steam penetration challenges outside those previously observed, should be analyzed using the multiple assessment criteria until sufficient data exists to identify similarities/dissimilarities relative to previously qualified commodities. Once this characterization is completed, future studies may then rely on thermometric lethality data.
The methods described in this article result in a sterilization and documentation control system where
-
A controlled version of a batch preparation list is developed and used during manufacturing. This list is attached to preparation procedures with items not separated out by specific load but listed individually. The load they are autoclaved in is documented on the batch preparation list form during routine operation and included with batch documentation.
-
The approved component list can be maintained through the site change control program, and new items can be added. The list would also serve as a part of the annual sterilization validation summary for the site.
-
For non–batch-specific loads, a separate form was created allowing items to be written in so as to document what went through the autoclave. The non–batch-specific form is stored in an equipment file tracking everything processed through that autoclave.
-
For loads or items requiring assembly, the assembly instructions and configurations were captured in standard operating procedures or preparation instructions for assembly prior to steam sterilization, for example, a pump assembly that includes the product supply manifold, pumps, filling nozzles, and associated tubing.
-
The validation would be run using a minimum load (e.g., cart and a piece of tubing) and a maximum load and then combinations of different items to show consistency among configurations.
-
A maximum load based on mass was validated to establish an item and load bracket of parts. This established the maximum quantity of each item validated, provided the personnel adhere to the preparation and loading orientation/configuration criteria to facilitate air removal and steam penetration. This would be the identified load for annual requalifications.
-
Based on the validation, a master list of maximum items in the load can be maintained and used to assess new product or component configurations being introduced to the facility.
The result of the item mapping and load bracketing data is within “a proven acceptable range” of sterilizing conditions. The critical control points for maintenance of these conditions are the component preparation procedures, commodity orientation in the load, commodity proximity to other items allowing for adequate steam penetration, and the maximum allowable load size based upon mass. These sterilization conditions result in items that are sterile, functional, allow for operational flexibility, and do not compromise product sterility.
Acknowledgments
We wish to acknowledge Ray Beery (BVS), Greg Lynch (ECVS, Inc.), and Jim Anglin for providing much of the data discussed in this article. Special thanks to Bill Jones (HGS) for technical review and input. This information was previously presented at the 2006 PDA annual meeting in Anaheim, California.
- © PDA, Inc. 2010