Abstract
It was recently found that after storage of a live viral vaccine at −80 °C in glass vials closed with rubber stoppers, a phenomenon was revealed which had not been observed before with other viral products stored at −20 °C: overpressure in the vials. As this phenomenon poses a serious safety problem for medical personnel as well as for the product itself, an investigation was initiated to identify the root cause of the overpressure. After exclusion of possible root causes (differences in air temperature or atmospheric air pressure during filling and quality control testing, outgassing from the formulation buffer) the remaining hypothesis involved a possible container closure integrity issue at low temperature. The glass transition temperatures (Tg) of many rubber stopper formulations are in the range −55 to −70 °C. At storage temperatures below Tg, the rubber stopper loses its elastic properties and there is a risk that the seal integrity of the vial could be compromised. Loss of seal integrity of the vials near storage temperatures of −80 °C would result in an ingress of cold dense gas into the vial headspace. After removal of the vials from storage at −80 °C, the rubber stoppers could regain their elastic properties and the vials would quickly reseal, thereby trapping the ingressed gas, which leads to overpressure in the vial headspace. Nondestructive laser-based headspace analysis was used to investigate the maintenance of container closure integrity as a function of the filling and capping/crimping process, storage and transport conditions, and vial/stopper designs. This analytical method is based on frequency modulation spectroscopy (FMS) and can be used for noninvasive headspace measurements of headspace pressure and headspace gas composition. Changes in the vial headspace composition and/or pressure are a clear marker for vials that have lost container closure integrity.
LAY ABSTRACT: After storage of a live viral vaccine at −80 °C in glass vials closed with rubber stoppers, overpressure in some of the vials was observed, posing a serious safety problem for medical personnel as well as for the product. A working hypothesis to explain this phenomenon involved a possible container closure integrity issue at these low temperatures. The glass transition temperatures (Tg) of many rubber stopper formulations are in the range −55 to −70 °C. At storage temperatures below Tg, the rubber stopper loses its elastic properties, resulting in compromised seal integrity of the vial and ingress of cold dense gas into the vial headspace. Upon thawing, the rubber stoppers regain their elastic properties and the vials quickly reseal, thereby trapping the ingressed gas, which leads to overpressure in the vial headspace. Nondestructive, laser-based headspace analysis, which is able to detect changes in headspace pressure and gas composition, was used to investigate the maintenance of container closure integrity. Changes in the vial headspace composition and/or pressure are a clear marker for vials that have lost container closure integrity.
- Storage at −80 °C
- Live viral vaccines
- Container closure integrity
- Headspace analysis
- Frequency modulation spectroscopy
- Glass transition temperature
Introduction
A storage temperature of −80 °C for a live viral vaccine (or any other pharmaceutical product) introduces many practical challenges, is expensive, and should be avoided whenever possible. Nethertheless, during clinical testing, a storage temperature of −80 °C is often chosen for live viral vaccines because stability data are not available to support product storage at higher temperatures for the required shelf life.
It was recently found that storage of a live viral vaccine at −80 °C in glass vials closed with rubber stoppers revealed a phenomenon which had not been observed before with other viral products stored at −20 °C: overpressure in the vials. During quality control (QC) testing, vials stored at −80 °C were thawed to room temperature (RT) and the rubber stopper was punctured with the needle of a syringe. In approximately 20–40% of the vials (percentage depending on the product batch), the syringe piston moved backwards upon insertion of the needle. After withdrawal of the needle, vaccine product shot out of the needle insertion hole indicating a considerable overpressure within the vials. For some vials the cap was removed completely which allowed the stopper to pop up from the vial. As this phenomenon posed a serious safety problem for medical personnel as well as a potential sterility risk for the product itself, an investigation was initiated to identify the root cause of the overpressure.
A couple of possible root causes were identified and could be excluded after thorough investigation. Differences in air temperature or atmospheric air pressure during filling and QC testing could result in pressure differences of only a few mbar, much lower than the overpressure actually observed. Identity testing of the formulation buffer confirmed that the specified ingredients and the potential for chemical reactions causing outgassing from the formulation buffer could be ruled out.
After exclusion of these possible root causes, the remaining hypothesis involved a possible container closure integrity issue at low temperature. The glass transition temperatures of many rubber stopper formulations are in the range −55 °C −70 °C. At storage temperatures below the glass transition temperature, the rubber stopper might lose its elastic properties with risk that the seal integrity of the vial could be compromised. Loss of seal integrity of the vials near storage temperatures of −80 °C would result in an ingress of cold dense gas into the vial headspace. After removal of the vials from storage at −80 °C, the rubber stoppers could regain their elastic properties and the vials would quickly reseal, thereby trapping the ingressed gas. This would lead to overpressure in the vial headspace due to volume increase of the trapped cold gas as it warms up.
To further investigate a possible issue with container closure integrity at a storage temperature of −80 °C as the root cause for overpressure in the viral vaccine product vials, four sets of experiments were performed. Nondestructive headspace analysis was used to investigate the maintenance of container closure integrity as a function of the filling process, storage and transport conditions, and vial/stopper packaging components. Asselta et al. (1) previously identified laser-based headspace analysis as an effective means to identify closure integrity failures at low temperatures. This analytical method is based on frequency modulation spectroscopy (FMS) and can be used for noninvasive headspace measurements of headspace pressure and headspace gas composition. A number of previous publications have described the use of FMS to characterize the headspace in sterile pharmaceutical product vials for various applications (2⇓⇓–5). Of particular interest for this study, changes in the vial headspace composition and/or pressure are a clear marker for vials that have lost container closure integrity (2). Analytical platforms based on FMS can therefore be implemented to perform a quantitative physical container closure integrity test. The nondestructive nature of the measurement enables 100% analysis of product as well as the ability to measure a single sample over multiple time points.
Materials and Methods
The materials listed in Table I were used for the investigations.
Various Vial Stopper Combinations Using the Components Listed in This Table Were Used in Container Closure Studies after Storage at −80 °C
Buffer Sample and Vial Closure Preparation
In the first set of experiments, the vials were filled either manually or by use of an automated filling line with 0.7 mL of different types of buffers, either at RT or at +5 °C. Stoppering and crimping of both manually- and automatically-filled vials was done automatically (see description below). For all subsequent sets of experiments, the vials were prepared in a way mimicking the filling of the actual viral vaccine product as closely as possible. Formulation buffer or saline was stirred at RT and filled in portions of 0.7 mL into the vials by use of an automated filling line. The vials were crimped using an automated system with capping and crimping parameters that were set manually. In the course of experiments two settings were used: stiff and tight. The stiff setting barely allowed manual movement of the cap whereas the tight setting did not allow any movement of the cap after crimping. For the rest of this paper, samples crimped using the stiff setting will be referenced as loosely crimped while samples crimped using the tight setting will be referenced as tightly crimped.
Low-Temperature Storage
For the first set of experiments, filled vials were stored at RT, +5 °C, −20 °C, −80 °C and on dry ice and analysed after 13 days of storage. This period of time was chosen for subsequent investigations as the product vials which initially showed the phenomenon of overpressure in QC testing were stored at −80 °C for a period of 13 days. Prior to the measurement, vials were allowed to equilibrate to RT in normal air for at least 1 h.
For storage at −80 °C, a deep freezer was used. In the second set of experiments, the cold transport process of product vials was mimicked by storing and shipping sample vials on dry ice followed by subsequent headspace analysis of the samples.
The last two sets of experiments used vials shipped at RT and stored on dry ice for defined periods as described in the Results section. Prior to headspace oxygen analysis, vials stored on dry ice were allowed to equilibrate to RT in a carbon dioxide-rich atmosphere to prevent any gas exchange with normal air during thawing.
Headspace Analysis
Headspace oxygen measurements were performed using a nondestructive headspace oxygen analyzer (model FMS-760, Lighthouse Instruments, Charlottesville, VA, USA). Calibration was performed using certified 20% and 0% oxygen standards.
These certified standards were made by backfilling 2R vials with certified NIST traceable oxygen gas mixtures and then flame sealing them shut. Because they are made from 2R vials, the standards can be measured identically to 2R vial samples. The results listed in Table II are of repeated measurements of the known standards and demonstrate the accuracies and precisions of the headspace oxygen measurements for the 2R vial configuration.
Results of Five Consecutive Measurements on Certified Standards of Known Oxygen Concentration
Five consecutive measurements were made on each of six known oxygen standards to verify the performance of the system (see Table II for an example of headspace oxygen performance data). The headspace oxygen in each sample vial was then measured and recorded.
Headspace pressure measurements were performed using a nondestructive headspace pressure analyzer (model FMS-1400, Lighthouse Instruments, Charlottesville, VA, USA). Calibration was performed with certified pressure/moisture standards.
These certified standards were made by evacuating 2R vials to known calibrated pressures and then flame sealing them shut. Because they are made from 2R vials, the standards can be measured identically to 2R vial samples. The results listed in Table III are of repeated measurements of the known standards and demonstrate the accuracies and precisions of the headspace pressure measurements for the 2R vial configuration.
Results of Three Consecutive Measurements on Certified Standards of Known Pressure Levels
Three consecutive measurements were made on each of seven known pressure standards to demonstrate the performance of the system (see Table III for an example of headspace pressure performance data). The headspace pressure in each sample was then measured and recorded.
The samples were allowed to thaw for at least one hour before the headspace analysis. For cases where the samples were stored in a deep freezer (air environment), the samples were thawed to RT in an air environment. For cases where the samples were stored on dry ice (carbon dioxide environment), the samples were allowed to thaw in a carbon dioxide–rich atmosphere. This was done by removing the samples from the storage box with dry ice and placing them into a 25 L plastic container. The lid of the plastic container was sealed with a rubber O-ring and had two holes, one hole for a rubber tube connected to a carbon dioxide gas source, the other hole for a rubber tube that served as an exhaust. When the samples were placed inside the plastic container, the carbon dioxide gas source was opened and the container was purged continuously for 15 min with carbon dioxide at a rate >5 SLPM (standard liters per minute). After 15 min of purging, the holes of the container lid were sealed with tape and the samples were allowed to thaw in the carbon dioxide-rich atmosphere for a minimum of 1 h.
Results and Discussion
The objective of the first set of experiments was to confirm the overpressure phenomenon. After the initial observation of the overpressure phenomenon, a number of viral vaccine product vials from four different batches were investigated for overpressure after storage at different temperatures (Table IV).
Overpressure Results (≥1500 mbar) of Viral Vaccine Product Vials Stored at Different Temperature Conditions
No vials with overpressure were identified after storage at +5 °C and −20 °C. The number of vials with overpressure after storage at −80 °C or −80 °C/dry ice differs remarkably between batches 1 and 2 and batches 3 and 4. As part of the first set of experiments it was then decided to investigate the influence of the storage temperature, filling temperature, and vial design on the potential creation of overpressure vials. In addition, three similar compositions of the formulation buffer from two different vendors were investigated for outgassing of buffer components as a possible root cause for the observed overpressure phenomenon. A total of 769 vials were filled either automatically or manually with 0.7 mL of different types of buffers, either at RT or at +5 °C. Two different types of 2R vials from the same manufacturer were used, non–blow back (VNB) and blow back neck design (VBB). For all fillings, the same lot of stoppers was used from manufacturer 1, a non–blow back design (SNB1). For details, see Table V. The buffer-filled vials as well as the viral vaccine product vials were crimped loosely (see Materials and Methods).
Composition/Production of Buffer-Filled Vials for First Set of Experiments
Filled vials were stored at RT, +5 °C, −20 °C, −80 °C and on dry ice for 13 days. Prior to the headspace pressure measurements, vials were allowed to equilibrate to RT. In contrast to the initially measured viral vaccine product vials, none of the prepared buffer vials displayed overpressure, even after storage at −80 °C and on dry ice.
To help explain these results, a closer look was taken at the packaging components used for the vials in the first set of experiments. For all investigated vials the same design of stoppers (non–blow back, SNB1) and vials (blow back, VBB) were used. This combination of non–blow back stoppers and blow back vials had been chosen to limit the number of required primary packaging components for the studies as these vials and stoppers are also used for other freeze-dried viral vaccines. The suitabilty of this vial/stopper combination for liquid frozen vaccines stored at −20 °C had been proven for previous liquid products.
The type and lots of vials and stoppers used for the first set of experiments are listed in Table VI. From Table VI it can be seen that for product batches 1 and 2, a different lot of stoppers (A) and vials (C) were used than for product batches 3 and 4 (stopper lot B, vial lot D). This might explain the difference in the number of overpressure vials between batches 1 and 2 and batches 3 and 4. For the buffer-filled vials, the same lot of stoppers (B) and vials (D) were used as for batches 3 and 4. These results indicate that the different lots of vials and stoppers may be partly responsible for the overpressure phenomenon. However, the fact that none of the buffer vials showed overpressure motivated further sets of experiments to investigate additional factors besides the lots of vials and stoppers that could potentially be responsible for the overpressure phenomenon.
Listing of the Different Types and Lots of Vials and Stoppers Used for Four Batches of Live Viral Vaccine Product and Various Buffer-Filled Vials
In order to verify how vial closure integrity is dependent on the vial/stopper combination, a second set of experiments was performed. In total, 10 different vial/stopper combinations were tested, all of them crimped loosely (see Materials and Methods). Two different designs of the 2R vials were chosen from the same manufacturer, non–blow back (VNB) and blow back design (VBB). Different designs of the stoppers were identified, from manufacturer 1, non–blow back (SNB1) and blow back design (SBB) and from manufacturer 2, non–blow back design (SNB2). Besides the different designs, different lots of vials were checked for size of the inlet diameter (Table VII) and different lots of stoppers were checked for size of the plug diameter (Table VIII). The diameters of the vials and stoppers are derived from the incoming goods control of the respective lots. The average diameters are calculated from 20 single measurements.
Inlet Dimensions of Vials from Various Lots
Stopper Plug Dimensions of Various Lots of Stoppers
The vial lots (blow back) with the largest and smallest inlet diameter were chosen for investigation, as well as one lot of non–blow back vials. A non–blow back stopper lot from manufacturer 1 with the largest and smallest plug diameter was identified for subsequent investigations, as well as a non–blow back stopper from a different manufacturer 2 (SNB2), because the specified stopper plug diameter from manufacturer 2 is approximately 0.1 mm larger than the specified stopper plug diameter from manufacturer 1. In addition, a blow back stopper from manufacturer 1 was chosen for subsequent investigation. Altogether, 3 different vials and 4 different stoppers were investigated in 10 different vial/stopper combinations (Table IX).
Overview of Vial/Stopper Combinations Tested in the Second Set of Experiments, Vials Identified after 14 Days Storage on Dry Ice with Depleted Headspace Oxygen Levels
A total of 50 vials of each vial/stopper combination were filled with 0.7 mL of saline, and stoppered and crimped, using an automated filling line. After storage for 14 days on dry ice, vials were thawed in a CO2 atmosphere and measured for oxygen content. If vials are stored at dry ice temperature (−78.5 °C), the initial 1 atm headspace pressure of the vials at RT is significantly reduced due to the cooling of the initial air headspace. If rubber stoppers lose their seal integrity at these low temperatures, the pressure gradient drives CO2 into the headspace through the leak displacing some or all of the original air headspace.
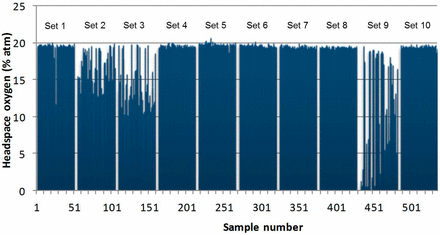
Figure 1 shows the measured headspace oxygen results after 14 days of storage on dry ice. Four of the vial stopper combinations (combinations 1, 2, 3, and 9) have produced samples with depleted oxygen levels (<17% oxygen) after storage on dry ice with combinations 2, 3, and 9 having especially large numbers of oxygen-depleted vials; this is summarized in Table IX. The use of the blow back stopper from manufacturer 1 in combination with all three different vial types (sets 1, 2, and 3) resulted in multiple instances of oxygen depletion. Sets 1 through 3 show 4, 27, and 39 vials with depleted oxygen levels, respectively. The vial/stopper combination previously used as the standard combination did not maintain container closure integrity at −80 °C (set 9: 31 vials with depleted oxygen level), if the combination large vial/small stopper was used. In contrast, the combination small vial/large stopper (set 7) of the standard combination did not show any vials with depleted oxygen levels, strengthening the hypothesis that design as well as size of vials and stoppers are important for storage at −80 °C.
Headspace oxygen results from measurements of all samples from 10 different vial/stopper combinations in the second set of experiments.
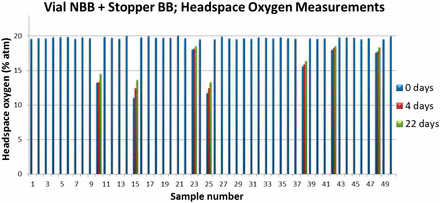
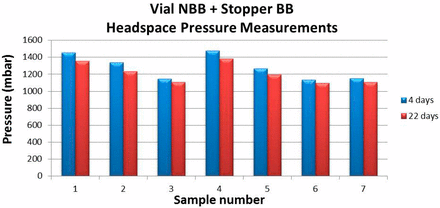
After the initial measurements, vials identified as having depleted levels of headspace oxygen were stored at RT in a normal air environment and measured again after 4 and 22 days to monitor the time evolution of the headspace oxygen content. The time-evolved measurements also included measurements of the headspace pressures. The objective of the time-evolved measurements was to correlate depleted oxygen levels in a vial to the presence of overpressure, and to determine if leaks in the samples were temporary or permanent. As an example of the results, Figure 2 and Figure 3 plot the time evolved headspace measurements for vial/stopper combination 1. In addition to the four samples having oxygen levels <17%, three additional samples having slightly depleted oxygen levels (between 17% and 18%) were also monitored. After a 22 day period, vials that were initially identified as having depleted oxygen levels showed a slight increase in headspace oxygen. Headspace pressure measurements confirmed that the oxygen depleted vials also contained overpressure. The overpressures in the headspace were also monitored over time and the results in Figure 3 show a slight decrease in overpressure over this time period.
Time-evolved headspace oxygen results from measurements of oxygen-depleted samples from vial/stopper combination 1.
Plot of time-evolved headspace pressures measured in oxygen-depleted vials from vial/stopper combination 1.
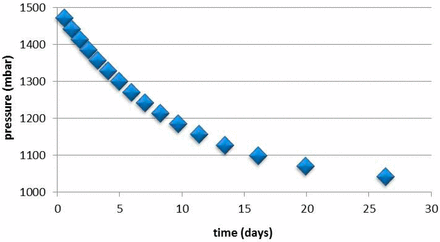
To help interpret the time-evolved measurements, comparisons can be made to calculations of a headspace leak rate model described in (6). This headspace leak-rate model has been validated with experimental studies and allows the calculation of changing headspace conditions due to a leak defect. Input parameters for the model are headspace volume, effective leak hole size, and initial headspace conditions. Figure 4 demonstrates how quickly an overpressure of 1500 mbar in a 2R vial having an empty headspace volume of 3 mL would come to equilibrium with an atmosphere environment assuming a very small effective leak hole size of 0.2 μm. The rate of pressure change in the graph of Figure 4 can be compared to the rate of pressure change measured in the samples as plotted in Figure 3. One can conclude that the change in pressure of the samples (less than 100 mbar within 18 days) is minor compared to that calculated by the headspace leak rate model for a 0.2 μm leak (approximately 400 mbar within 18 days). These results indicate that the samples are sealed and are maintaining the overpressure. There is no sizable leak present in the sample vials during storage at RT which allows equilibration of the headspace overpressure. The fact that the oxygen levels do not appreciably increase over time due to an ingress of air through a leak as predicted by the headspace leak rate model is a second verification that the oxygen-depleted/overpressure vials have suffered temporary leaks during storage at low temperature and then reseal when warming up to RT.
Loss of overpressure for 2R vial, 0.2 μm leak (leak rate model).
As could be seen in the second set of experiments, container closure integrity at −80 °C depends on the design as well as on the relative size of the vial neck and the stopper plug. This is a noteworthy observation as it is generally accepted that the most robust seal is created by the stopper flange and the top vial finish, provided that the aluminum crimp is applied with sufficient pressure. The non–blow back stopper from manufacturer 2 (SNB2) was chosen for all future applications, because the stopper plug diameter is approximately 0.1 mm larger compared to the plug diameter of the non–blow back stopper from manufacturer 1, thereby enhancing the chances of obtaining appropriate container closure integrity at −80 °C due to size of the stoppers. In addition, the Tg of the rubber stopper from manufacturer 2 is approximately 5 °C lower than the Tg of the rubber stopper from manufacturer 1.
A third set of experiments was planned and executed to identify the onset of overpressure as a function of storage time. Three slightly different lots of vials and one lot of the non–blow back stopper from manufacturer 2 were investigated. The vial stopper combination from set 9 described above in Table IX (large vial/small stopper) was used as a positive control, where the phenomenon of overpressure vials could be expected. Three hundred vials of each of the four vial/stopper combinations were filled with 0.7 mL formulation buffer, and stoppered and crimped, using an automated filling line. Vials were stored on dry ice for 3, 6, 14, and 21 days. After equilibration to RT in a CO2 rich atmosphere, vials were measured for oxygen content and overpressure.
No oxygen-depleted samples (samples having <17% oxygen) or samples with overpressure (samples having headspace pressure >1150 mbar) were identified after day 3 and day 6 measurements in all of the four vial/stopper combinations, including the positive control vials. After 14 and 21 days, respectively, one and three of the vials of the positive control (a different set of vials was measured at each timepoint) showed reduced oxygen content and overpressure. All other vial/stopper combinations using the stoppers from manufacturer 2 were without overpressure vials. A summary of these results is shown in Table X.
Summary of Results of Third Set of Experiments
The results of the third set of experiments are in strong contrast to the second set of experiments, where set 9, the same vial/stopper combination used as the positive control in the third set of experiments, showed 31 out of 50 vials with overpressure. The failure rate has dropped dramatically for this combination in the third set of experiments compared to the second. Upon further examination, the capping/crimping process was identified as possibly contributing to the root cause for the overpressure effect in addition to the design and size of vials and stoppers. The vials in this third set of experiments were crimped tightly, whereas the vials of the previous experiments were crimped loosely (see Materials and Methods). This is again a noteworthy observation. Using a higher crimp pressure is not sufficient to maintain a robust seal between the stopper flange and the top vial finish and eliminate container closure integrity failure at −80 °C storage. Only the addition of the extra sealing surfaces from a relatively large stopper plug provides reliable seal integrity.
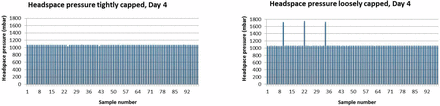
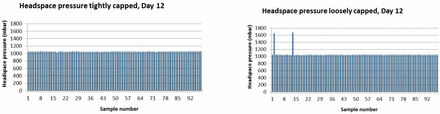
To investigate this hypothesis further, a fourth set of experiments was performed using the vial/stopper combination non–blow back vial and non–blow back stopper from manufacturer 2. This vial/stopper combination gave the best results in the previous studies due to the optimum relative dimensions of a small inner vial neck diameter and a large stopper plug diameter. A total of 392 vials were filled with 0.7 mL formulation buffer; half of the vials were “tightly crimped,” the other half were “loosely crimped.” The vials were measured for overpressure after 4 and 12 days of storage on dry ice. Figures 5 and 6 show the headspace pressure results.
Overpressure results for vials stored on dry ice for four days comparing samples capped and crimped with a tight setting to samples capped and crimped with a loose setting.
Overpressure results for vials stored on dry ice for 12 days comparing samples capped and crimped with a tight setting to samples capped and crimped with a loose setting.
After 4 days of storage on dry ice, the loosely crimped set showed three vials having an overpressure. After 12 days storage on dry ice, two overpressure vials were identified in the loosely crimped set (a different set of vials was measured at each time point). There was no vial with overpressure in the sets of tightly crimped vials. These results are summarized in Table XI and confirm that even an “optimal” vial/stopper combination from a design and dimensional point of view needs to be appropriately capped and crimped to maintain container closure integrity during storage at −80 °C.
Summary of Results Investigating the Influence of Capping and Crimping to the Loss of Container Closure Integrity during Cold Storage
Conclusions
The results of this study support the original hypothesis that overpressure found in product vials is a result of losing container closure integrity during cold storage. The storage of stoppered vials at a temperature of −80 °C increases the risk of compromising container closure integrity due to the fact that this temperature is below the glass transition temperature (Tg) of most rubber stopper formulations. At storage temperatures below Tg, the rubber stopper can lose its elastic properties and there is a risk that the seal integrity of the vial is compromised. This study also demonstrated that stoppered vials that lose closure integrity at low temperatures will reseal when warming back up to RT. Once the vial reaches a temperature above the Tg of the rubber stopper, the stopper regains its elastic properties and reseals the vial closure. Leaks at these low storage temperatures can therefore be temporary.
Some of the container closure integrity tests described in the Pharmacopeias and other sources (7⇓⇓–10) are not useful for identification of container closure integrity failures during storage at −80 °C. This is because these physical container closure integrity tests identify containers that are leaking at the time of measurement. Because of the impracticality of analyzing vials while they are stored at −80 °C, these measurements for container closure integrity are often performed at RT after the leaks have resealed. The measurement at RT does not reflect the status of the vials during storage at −80 °C and therefore does not detect the temporary leak.
On the other hand, a nondestructive analytical method that quantitatively characterizes the gas conditions of the vial headspace provides a way to detect vials that have been temporarily leaking at low storage temperatures. The headspace method, based on FMS technology, enabled studies described in this paper which identified the root cause of a headspace overpressure phenomenon in viral vaccine product vials stored at −80 °C. In addition, the studies demonstrated that the headspace method enabled identification of rubber stopper/vial combinations that maintain closure integrity at storage temperatures below the glass transition temperature of the rubber stoppers. Critical factors for the maintenance of container closure integrity included appropriate design of the vial and stopper plug, relative dimensions of the stopper and the vial neck giving a tight fit, as well as an appropriately tight capping and crimping process. The dimensional variability found between different vial and rubber stopper lots as well as different specifications for the 13mm stopper depending on stopper manufacturer motivates a careful selection of packaging components for storage at −80 °C. For future consideration, rubber formulations with Tg below −80 °C might also have a positive effect on maintaining container closure integrity at deep frozen storage conditions.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgements
The authors thank all at Bavarian Nordic, IDT Biologika, and Lighthouse Instruments who contributed to solve this problem: identifying a measurement procedure for overpressure in vials, discussing results, designing experiments, and preparing all the materials for measurement.
- © PDA, Inc. 2012