Abstract
The evolutionary development of pharmaceutical transformation was studied through systematic review of the literature. Fourteen triggers were identified that will affect the pharmaceutical business, regulatory science, and enabling technologies in future years. The relative importance ranking of the transformation triggers was computed based on their prevalence within the articles studied. The four main triggers with the strongest literature evidence were Fully Integrated Pharma Network, Personalized Medicine, Translational Research, and Pervasive Computing. The theoretical quality risks for each of the four main transformation triggers are examined, and the remaining ten triggers are described.
LAY ABSTRACT: The pharmaceutical industry is currently going through changes that affect the way it performs its research, manufacturing, and regulatory activities (this is termed pharmaceutical transformation). The impact of these changes on the approaches to quality risk management requires more understanding. In this paper, a comprehensive review of the academic, regulatory, and industry literature were used to identify 14 triggers that influence pharmaceutical transformation. The four main triggers, namely Fully Integrated Pharma Network, Personalized Medicine, Translational Research, and Pervasive Computing, were selected as the most important based on the strength of the evidence found during the literature review activity described in this paper. Theoretical quality risks for each of the four main transformation triggers are examined, and the remaining ten triggers are described.
- Pharmaceutical transformation
- Quality risks
- Regulatory science
- Fully integrated Pharma network
- Personalized medicine
- Translational research
- Pervasive computing
- Open innovation
1. Introduction
The pharmaceutical industry since 1990 has experienced a decline in research and development (R&D) productivity, despite significant advancements in biomedical sciences and increasing R&D expenditure (1⇓⇓⇓⇓–6). According to the U.S. Food and Drug Administration (FDA), the problem exists because the current medical product development path is becoming increasingly challenging, inefficient, and costly. The FDA, in its 2004 landmark publication “Innovation/Stagnation” (7) illustrated that between 1993 and 2003 there was a significant drop in the number of new chemical and biologic applications submitted for approval. The FDA claims that this is because of the rising costs of product development that often force the innovators to focus their efforts on products with a potentially high market return (7).
To address the innovation problem and ongoing evolutions in the regulatory landscape (8⇓⇓⇓–12), the industry is making transformational changes to the pharmaceutical business. In this paper transformation is defined as the process by which the pharmaceutical industry intends to achieve and maintain advantage through changes in operational concepts, regulatory science, and technologies that will significantly improve its capability to innovate. The term regulatory science refers to the science of developing new tools, standards, and approaches to assess the safety, efficacy, quality, and performance of regulated medical products (based on the FDA definition; see http://www.fda.gov/scienceresearch/specialtopics/regulatoryscience/default.htm).
A key feature of the ongoing industry transformation is open innovation. This means that pharmaceutical companies no longer solely rely on their centralized and internally focused R&D and are increasingly looking towards external sources of innovation such as research partnerships with small biotechnology companies, universities, governmental organizations, and others (13⇓⇓–16). Since 2001, some of the large pharmaceutical companies such as Glaxo, Pfizer, and Lilly have experimented with the open innovation approach (17). Hunter and Stephens (17) see open innovation as “a valuable model for large pharmaceutical companies” and argue that adopting an open innovation culture will require a change in operational concepts, deployment of new technologies, and application of resources to nurture external collaborations and monitor their progress to ensure success.
Evaluation of the literature is a plausible method to characterize potential transformation triggers. The systematic discernment of patterns from a widely diverse set of studies and/or body of research requires analytical review (18). This study is a literature review focusing on qualitative content analysis of the primary articles with a systematic literature search (19) for selection of articles.
This paper is the second in a series that explores ongoing transformation in the pharmaceutical industry and its impact on pharmaceutical quality from the perspective of risk identification. The aim of this paper is to characterize pharmaceutical industry transformation triggers and associated theoretical quality risks via systematic review of literature.
2. Methodology
A six step process was followed, organized into three phases: article selection, article review, and article classification.
Step 1: Selection of primary articles
Step 2: Review of the primary articles
Step 3: Selection of derived articles
Step 4: Testing for article diversity
Step 5: Searching for transformation triggers in primary and derived articles
Step 6: Ranking of transformation triggers
The selection phase involved development of an article selection procedure (Step 1, Step 3). The review phase involved the detailed review of the primary articles to discern likely triggers for pharmaceutical transformation (Step 2, Step 5) and validation of article diversity (Step 4), and the classification phase was performed to determine relative importance ranking of the transformation triggers with respect to their prevalence within the articles studied (Step 6).
2.1. Selection of Primary Articles (Step 1)
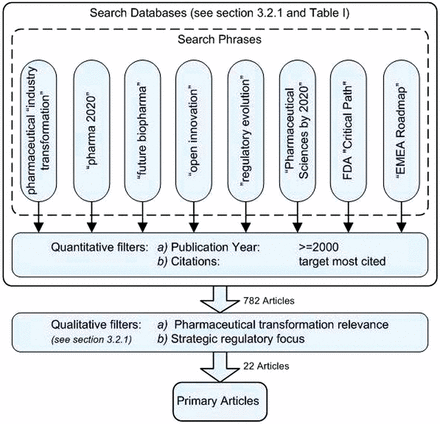
Transformational change in a given industry is influenced by regulatory policy, industry environment, and technological evolution (20). It is therefore important to design a selection procedure that taps into the diverse body of literature that reflects the balance between academic, industrial, and government-issued articles. The selection process for the primary articles was designed to achieve this goal and the procedure is described below. The inclusion criteria and search phrases listed in Table I was used in the search procedure to identify the primary articles. The primary article selection procedure is conceptually illustrated in Figure 1.
Inclusion Criteria and Search Phrases
Conceptual illustration of primary article selection.
2.1.1. Database and Search Phrases:
The main bulk of the article search activity took place during the first 9 months of 2010. Since the present research involves topics that span multiple disciplines such as pharmaceutical transformation, pharmaceutical innovation, pharmaceutical technology, and pharmaceutical regulatory sciences, the search tools used had to be diverse. For peer-reviewed academic articles Web of Knowledge, Science Direct, Wiley Online Library, and JSTOR were used. Regulatory agency websites—U.S. FDA, European Medicines Agency (EMA), Health Canada, and International Conference on Harmonization (ICH)—were the main source of regulatory articles. Articles published by the consulting firms and other research organizations were collected through general Google web search or Google Scholar. The Google Scholar search was limited to articles in business, medicine, pharmacology, and social sciences subject areas. Search phrases listed in Table I were used without truncation and in quotes when shown.
2.1.2. Publication Year:
The articles for each transformation trigger were selected from a time period that was as recent as possible. This was done based on the assumption that more recent articles reflect better the current thinking of the academic, consulting, governmental, industry, and research organizations. The year 2000 was used as the cut-off date since the initial exploratory work on article selection revealed that most forward-looking opinions within the articles typically considered a 5 to 10 year time horizon.
2.1.3. Number of Citations:
This selection criterion represents the total number of citations per article. Frequency of citation of an article is a sign of its pervasiveness and hence its ability to influence current and future thinking. Its value is affected by the year of publication. Therefore a balance between recentness (publication date) and pervasiveness (number of citations) was needed and was designed into the ranking procedure (see section 2.6). If available the citation information was gathered from the publisher of the source journal; otherwise the Google Scholar was used to determine the number of citations.
2.1.4. Pharmaceutical Transformation Relevance:
The literature search was focused on articles that had relevance to the healthcare sector in general and the pharmaceutical industry in particular. This search was performed by review of the abstract and/or the introduction section of the articles that had met the initial criteria (publication date, containing the word “pharmaceutical” or “healthcare” in the title or abstract) and therefore were considered interesting leads for further evaluation.
2.1.5. Strategic Regulatory Focus:
The initial exploratory article search revealed that within the United States and the European Union the current and future thinking of the regulators is often articulated in their long-term strategic plans. These plans dealt with the topics that are important from the perspective of public health protection and promotion. One such topic is creation of a regulatory environment that enables development of innovative, life-saving drugs, which regulators view important to public health promotion and use their long-term strategic plans to communicate their current achievements and future actions. For these reasons the last two search phrases listed in Table I were designed to discern articles that specifically dealt with forward-looking regulatory initiatives.
2.2. Review of the Primary Articles (Step 2)
Each primary article was reviewed in detail, meaning that the entire article was read focusing on views, opinions, actions, and evidence that provided clues on pharmaceutical industry evolution from an innovation and regulatory science perspective. Multiple occurrence of a particular topic relating to innovation or regulatory science was deemed important and was tagged as a transformation dimension. After review of all the primary articles (those selected during the initial search), the identified transformation dimensions were classified into similar groups which we have termed transformation triggers.
2.3. Selection of Derived Articles (Step 3)
During review of the primary articles any referenced articles within the text that were deemed relevant within the scope of the primary article were noted. Based on a brief review of the abstract and/or introduction section, a determination was made whether the relevant articles were compliant with the same inclusion criteria as for the primary articles, and if so they were considered as derived articles.
2.4. Testing for Article Diversity (Step 4)
It was necessary to enhance generalization of results derived from the literature review to demonstrate that articles supporting each of the transformation triggers came from diverse sources but with similar sourcing characteristics, that is, article type, age, and by definition pervasiveness. This goal was primarily achieved through the design of the article selection procedure and was validated through descriptive statistics and application of the Kruskal-Wallis H-test (21).
2.5. Searching for Transformation Triggers in Primary and Derived Articles (Step 5)
Once the primary and derived articles were identified, the next step was to assess the presence of transformation triggers or closely related phrases in each of the articles. Content of each article was searched using the search phrases listed in Table II. Two or more search phrases were used for each transformation trigger. An article was deemed relevant to a transformation trigger if it covered at least one of the related search phrases listed in Table II. The following abbreviations were used to categorize the article types: academic (Acd), consulting (Con), government (Gov), industry (Ind), and research organization (Org).
Search Phrases Related to Transformation Triggers
2.6. Ranking of Transformation Triggers (Step 6)
In order to highlight the relative ranking of each transformation trigger, a weighted scoring approach was employed, similar to an approach described by Chan and Walmsley (21). To achieve this goal an importance weight score was applied to each attribute characterizing the article. The weighted score for each article with respect to each of the transformation triggers was computed based on the following criteria for the article attributes and the corresponding weighting scheme listed in Table III.
Weighing Scheme for Relative Importance Ranking of the Articles
Publication Source. Regulatory policy, the current thinking of the regulators, and their future plans are tangible examples of future regulatory direction and therefore were given the largest weight. Academic peer-reviewed articles by definition are thoroughly vetted and therefore were given the second largest weight. Articles written by renowned consulting organizations typically reflect and influence the key stakeholders in the industry and therefore received next priority weight. Articles written by industry practitioners not published in peer-review journals and non-Pharma research organizations receive the lowest weight.
Publication Year. Articles published before 2000 (Pre) or after 2000 (Post).
Number of Citations. The prevalence of the transformation triggers in the primary and derived articles was determined as described in section 2.5, and their relative ranking was performed in accordance with the computational procedure described in Table IV. The structure of the ranking matrix is illustrated in Table V (where rows are the primary and derived articles and columns are the transformation triggers).
Procedure for Computing Relative Importance Ranking of All Articles
Ranking Matrix
3. Results and Discussion
3.1. Results
The article selection procedure resulted in 22 primary articles targeted for literature review; and additional 60 articles were derived from review of the primary articles (see Table VI).
Article Search Phrases and Selection Results
Validation of article source diversity was achieved using descriptive statistics provided in Table VII and Kruskal-Wallis H-test for transformation triggers (HTT). The null hypothesis was defined to mean that most articles have similar sourcing characteristics, and the alternative hypothesis was defined to mean that most articles have diverse sourcing characteristics.
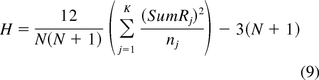 where
where
- Rj
- (Rank of each transformation trigger in Table VIII; where j = 1 … K)
- nj
- (number of data points per transformation trigger in Table VIII; where j = 1 … K)
- N = 146
- (total number of data points for all transformation triggers)
- K = 14
- (number of transformation triggers)
- df = (K − 1) = 13
- (degrees of freedom)
- HTT = 13.02
- (result of solving eq 9)
Since HTT = 13.02 is less than the chi-squared H-test table value of 19.812 (refer to standard chi-squared distribution table—not included) the probability of occurrence, that is, the P-value is greater than 0.10. Hence the null hypothesis is accepted and it can be concluded that most articles have similar sourcing characteristics—meaning most articles are academic in nature, published after 2005, with less than five citations.
Descriptive Statistics for Article Diversity
The qualitative assessment of views, opinions and evidence presented in the primary articles resulted in 14 transformation triggers. The relative importance ranking results for each of the transformation triggers are provided in Table VIII.
Presence of Transformation Triggers in Primary and Derived Articles and Relative Ranking
3.1.1. Results of the Literature Review:
Among the 14 transformation triggers, four triggers—namely Fully Integrated Pharma Network (Trigger 2), Personalized Medicine (Trigger 3), Translational Research (Trigger 5) and Pervasive Computing (Trigger 14)—were found to be the most prevalent within the articles studied. The results of the literature review are discussed below. Note that the ordinal positioning of the transformation triggers in Table VIII (1 to 14) is different from their importance ranking provided at the end of the table. The 14 triggers are simply listed in the order of transformation topics that were addressed during the literature search and review process: organization, product, regulatory, and technology.
3.2. Discussion
It is important to note that the intent of the literature review was not to compare and contrast opposing views and opinions. Instead our intent was to perform a systematic review of the literature to obtain information from selected articles in order to identify likely triggers influencing pharmaceutical industry transformation. Information within the articles relating to transformation triggers were synthesized into blended statements that are presented below for each of the triggers. In most cases these blended statements are derived from multiple referenced articles and therefore their citation is presented as a cluster of articles. When the referenced articles apply to the entire section they are presented at the end of that section. The proposed open innovation trends and the theoretical quality risks for each of the four main transformation triggers are listed and discussed below.
Proposed theoretical quality risks are the following:
Effective oversight of research and commercial partnerships
Effective transfer of in-licensed products
Paucity of multifaceted regulatory knowhow in relation to combination products
Efficacy of the existing validation approaches to handle pervasive computing paradigm from the perspective of regulatory compliance
Effective management of sharing research data/information and outsourcing of data management activities from the perspective of regulatory compliance.
3.2.1. Trigger 2: Fully Integrated Pharma Network:
A major theme within the literature points to a pharmaceutical business model based on a fully integrated global network that includes other pharmaceutical or biotechnology companies, universities, organizations, and even individuals in some cases (11, 22⇓⇓⇓⇓–27). The open innovation trends for this transformation trigger will likely affect selection and employment of external research and commercial partnerships and in-licensing of products. The resultant theoretical quality risks will require establishment of effective due diligence and product transfer processes to mitigate the potential risks.
3.2.2. Trigger 3: Personalized Medicine:
The literature (28⇓⇓⇓⇓–33) points to the likely trends that specific treatments and therapeutics best suited for an individual are increasing in prevalence. There is no single definition for personalized medicine, but one general theme among the articles suggests that personalized medicine is concerned with the development and administration of treatments (based on a knowledge of genetic biomarkers or mutations) to patients who might best respond to an individually tailored treatment (28⇓⇓⇓⇓–33). This is exemplified by a quote from Adams et al. (33): “By 2015, a 21-year-old could undertake a whole genome test to identify risk factors for chronic conditions, such as a specific cancer or heart disease. It would also reveal the potential for adverse drug reactions to drugs. This knowledge will enable a new level of consumer responsibility.”
The open innovation trends for this transformation trigger will likely influence research, development, manufacturing, distribution, marketing, and surveillance of combination, biological, and biotechnology products. The resultant theoretical quality risks will require provision of multidisciplinary regulatory knowledge and skills to mitigate the potential risks.
3.2.3. Trigger 5: Translational Research:
The referenced articles (3, 26, 30, 34⇓⇓⇓⇓⇓–40) describe likely trends in translational research and define it as a bidirectional sharing of knowledge and ideas by the scientific and clinical disciplines to develop diagnostics that reliably select the mechanisms leading to breakthrough therapeutics. Some of the benefits argued by the articles include matching patients with therapy, improved compliance with therapy, reduced drug development costs, and reduced healthcare costs. Advances in computational tools such as predictive biosimulation systems, in silico modeling techniques, and bioinformatics are also highlighted in some of the articles as playing a key role in enabling the realization of the translational research (3, 26).
The open innovation trends for this transformation trigger will likely affect research partnerships and research information sharing. The resultant theoretical quality risks will require establishment of effective due diligence for research partnerships and provision of robust data management policies and procedures to mitigate the potential risks.
Trigger 14: Pervasive Computing:
The referenced articles (41⇓⇓⇓⇓⇓⇓⇓–49) characterize pervasive computing as an environment saturated with computing and communication capability. Smart medication packaging, tiny wireless sensors implanted on the patient body to monitor various vital signs, and remote monitoring devices to determine how patients respond during clinical trials are just some examples. Another pervasive aspect of computing is provision of externally hosted services for management of data (e.g., clinical, manufacturing, product surveillance, etc.) and associated technical infrastructure. The concept is oftentimes referred to as cloud computing (50⇓⇓–53), which is a computing model consisting of services that are commoditized and delivered in a manner similar to traditional utilities such as water, electricity, gas, and telephony. In such a model, users access services based on their requirements without regard to where the services are hosted or how they are delivered.
The open innovation trends for this transformation trigger will likely result in prevalence of smart, implantable devices for product tracking, patient monitoring, and drug delivery and in outsourcing of information systems for management of clinical and product data (e.g., for clinical trials, drug safety surveillance, customer complaints, etc.). The resultant theoretical quality risks will require establishment of effective validation procedures to ensure reliability of smart devices and provision of data management procedures to ensure security and integrity of outsourced data to mitigate the potential risks.
3.2.1. Brief Summary of Other Transformation Triggers Trigger 1: Healthcare Management–Focused:
The main thrust of the discussions in the referenced articles seem to suggest that pharmaceutical industry is transforming from a mainly product-based industry to a healthcare management concept with more emphasis on preventative and lifestyle medicine and associated services. It is anticipated that the industry will integrate a larger health offering with sustainable pricing models for a wider array of products and services, including generics, diagnostics, disease management, prevention, and knowledge management (4, 54, 55). From the perspective of quality risk management these novel and complex products, which require convergence of multiple scientific and technological disciplines, will challenge the regulators, industry, and healthcare professionals in their safe and effective use.
Trigger 4: Virtual R&D:
The main argument made by referenced articles (15, 24, 35, 56⇓–58) is that large pharmaceutical companies are shifting investment away from traditional in-house research activities and focusing more on developing superior deal-making and alliance capabilities to enable virtual R&D, which is also linked to the concept of open innovation.
Trigger 6: Adaptive and In-life Trials:
In adaptive trials, information acquired during a particular clinical trial is used to alter the course of the trial without compromising its statistical validity. In-life testing will leverage emerging computation and communication technologies and could replace Phase III trials. Such measures could shorten the developmental pipeline from the current 10 to 12 years to between 3 and 5 years. Closer relationship with regulatory authorities is a key factor to ensure success (3, 59⇓⇓⇓–63).
Trigger 7: Global Harmonization:
Harmonization discussions focus mainly on collaboration between regulators and the industry, especially in the ICH zone (North America, Europe, and Japan). Referenced articles include predictive statements hoping for a level of global harmonization that may ultimately result in the seemingly unattainable goal of having one application per trial to all authorities (8, 10, 64⇓–66).
Trigger 8: Science- and Risk-Based Regulations:
The articles examined agued that with the fates of the regulators and the industry more intertwined than ever, public health depends on regulatory innovation as much as on scientific progress. From the perspective of regulatory innovation, an important step towards achieving the outlined goal involves international collaboration between regulators and industry, which has been exemplified through ICH efforts manifested in issuance of a wide range of standards, particularly those related to quality risk management, pharmaceutical development, and pharmaceutical quality systems (8, 10, 53, 67⇓⇓–70).
Trigger 9: Live Licensing:
Discussions on this topic mainly have a conceptual tone due to uncertain commitment from the regulatory bodies. According to the literature, live licensing implies that the current Phase I to IV clinical testing process may eventually be selectively or wholly replaced by a system known as in-life testing or live licensing. Those proposals involve cumulative testing of the drug throughout its lifecycle. In this paradigm the industry would continually test drugs with smaller, more focused clinical trials. If a trial shows efficacy and safety, a live license would be given, allowing the company to market the drug in a limited manner (71⇓⇓⇓–75).
Trigger 10: Enforcement:
Articles studied anticipate a substantial increase in regulators' compliance and enforcement actions, particularly in the oversight of inspections, product promotion, and unapproved drugs (62, 76, 77).
Trigger 11: Biotechnology:
The recent applications of biotechnology will drive medical breakthroughs that will enable the people to improve their health and increase their longevity dramatically. To exploit the potential of biotechnology and emulate successes of the biotech companies, large Pharma will likely structure themselves as a collection of biotechnology sites that compete with each other and external biotechnology companies to supply compounds into a centralized development organization (78⇓⇓–81).
Trigger 12: Nanomedicine:
Generally the referenced articles (82⇓⇓–85) point to increasing use of nanobiotechnology by the pharmaceutical and biotechnology industries. Technical achievements in nanotechnology are being applied to improve drug discovery and pharmaceutical manufacturing. Some argue that in the near future, it might be possible to accurately model the structure of an individual cell and to predict its function using computers connected to nanobiotechnology systems. These futuristic statements imply that the detailed virtual representation of how a cell functions might enable scientists to develop novel drugs with unprecedented speed and precision, without doing any experiments in living animals.
Trigger 13: Bioinformatics:
Referenced articles (10, 30, 53, 86⇓–88) largely focused on application of information technology and computer science to the field of molecular biology. Some also focused on bioinformatics from a regulator's perspective, implying that it involves use of modern computer systems to effectively manage the regulatory product information supply chain.
4. Conclusions
Systematic review of the literature has enabled identification of 14 factors—referred to as transformation triggers—that influence the ongoing transformation in the pharmaceutical industry. The importance ranking of these factors reveal that of the 14 transformation triggers four, namely Fully Integrated Pharma Network (Trigger 2), Personalized Medicine (Trigger 3), Translational Research (Trigger 5), and Pervasive Computing (Trigger 14), are considered as the most impactful. Theoretical assessment of these four triggers from an open innovation and quality risk management perspectives by the authors has resulted in the following proposals that require validation and further research.
We propose that the open innovation trends in the pharmaceutical industry will likely increase with a particular impact on (i) external research, commercial partnerships, and in-licensing of products; (ii) research and development on combination, biological, and biotechnology products; and (iii) smart, implantable devices for product tracking, patient monitoring, drug delivery, and outsourcing of information systems for management of clinical and product data (e.g., for clinical trials, drug safety surveillance, customer complaints, etc.). These trends will in turn introduce unique quality risks that we argue should include the following risk topics and their associated mitigation strategies: (i) establishment of effective due diligence and product transfer processes, (ii) acquisition of multidisciplinary regulatory knowledge and skills, (iii) establishment of effective due diligence for research partnerships and for provision of robust data management policies and procedures, and (iv) establishment of effective validation procedures to ensure reliability of smart devices and provision of data management procedures to ensure security and integrity of outsourced data.
Limitations
A key limitation of this research is lack of similar, peer-reviewed studies for comparison purposes. This is a unique study, and the authors at the time of writing this paper did not find any published research with similar coverage. The next phase of the research is to determine the impact of the transformation triggers on pharmaceutical quality and validate the proposed theoretical risks by conducting an expert opinion survey via a questionnaire.
Conflict of Interest Statement
The authors declare that they do not have any financial or nonfinancial competing interests related to the content of this paper.
Acknowledgements
The corresponding author works for Sanofi (Bridgewater, NJ) and gratefully acknowledges the company's support for this research as part of his personal development. Views expressed in this paper are those of the authors and do not reflect the official policy or position of Sanofi.
- © PDA, Inc. 2013
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵