Abstract
An accelerating trend in the pharmaceutical industry is the use of plastic components in systems used to produce an active pharmaceutical ingredient or a finished drug product. If the active pharmaceutical ingredient, the finished drug product, or any solution used to generate them (for example, a process stream such as media, buffers, eluents, and the like) is contacted by a plastic component at any time during the production process, substances leached from the component may accumulate in the active pharmaceutical ingredient or finished drug product, affecting its safety and/or efficacy. In this article the author develops and justifies a semi-quantitative risk evaluation matrix that is used to determine the amount and rigor of component testing necessary and appropriate to establish that the component is chemically suitable for its intended use. By considering key properties of the component, the contact medium, the contact conditions, and the active pharmaceutical ingredient's or finished drug product's clinical conditions of use, use of the risk evaluation matrix produces a risk score whose magnitude reflects the accumulated risk that the component will interact with the contact solution to such an extent that component-related extractables will accumulate in the active pharmaceutical ingredient or finished drug product as leachables at levels sufficiently high to adversely affect user safety. The magnitude of the risk score establishes the amount and rigor of the testing that is required to select and qualify the component, and such testing is broadly grouped into three categories: baseline assessment, general testing, and full testing (extractables profiling).
LAY ABSTRACT: Production suites used to generate pharmaceuticals can include plastic components. It is possible that substances in the components could leach into manufacturing solutions and accumulate in the pharmaceutical product. In this article the author develops and justifies a semi-quantitative risk evaluation matrix that can be used to determine the amount and rigor of component testing that may be necessary and appropriate to establish that the component is suitable for its intended use. Use of the risk evaluation matrix allows a user of a component to determine the type and amount of testing that should be performed to establish the patient safety risk associated with using that component in order to manufacture an active pharmaceutical ingredient or a finished drug product.
Introduction
An accelerating trend in the pharmaceutical industry is the use of plastic, potentially “single-use”, components in systems used to produce an active pharmaceutical ingredient (API) or a finished drug product (FDP). This use of plastic components in the place of “traditional” production materials is driven by economic and quality considerations. Examples of such plastic components include tubing and connectors used to transfer starting buffers into the process stream, syringes used to transfer liquids, filters used with starting solutions, process intermediates and/or the final API (or FDP), bags (and associated tubing) used to store and transfer process-related solutions, tangential-flow filters used for concentration and diafiltration of process intermediates, chromatography resins and membranes used for the concentration and/or purification of process intermediates, o-rings used to seal connectors and sanitary fittings, final bulk containers, and sensors or other process-facilitating devices (1).
If any solution used to generate an API or FDP (for example, a process stream such as media, buffers, eluents, diluents, and the like) is contacted by a plastic component at any time during the API or FDP production process, the component and the solution may interact. It is well recognized in the pharmaceutical industry that interactions between such process stream solutions and the production system components must be controlled and minimized to ensure that the API or FDP is suitable for its intended use. The general requirements for production systems are contained in the guidelines published by several global regulatory agencies. In the United States, the requirements are enumerated in Title 21 of the Code of Federal Regulations, Part 211.65, which states that “equipment shall be constructed so that surfaces that contact components, in-process materials or drug products shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements” (2). In the European Union, a related statement is found in the EU Good Manufacturing Practice document, which states that “production equipment should not present any hazards to the product. The parts of the production system that come into contact with the product must not be reactive, additive or absorptive to such an extent that it will affect the quality of the product and thus present any hazard” (3). Additional guidance for APIs can be found in the ICH Q7 GMP Guidelines, which state that “equipment should be constructed so that surfaces that contact raw materials, intermediates or APIs do not alter the quality of the intermediates or APIs beyond the official or other established specifications” (4).
While all potential interactions between production equipment and the process stream solutions from which the API or FDP are derived are important in terms of the suitability of use of the API or FDP, this article focuses on the situation in which a substance from a production system component (extractable) leaches into a process solution and becomes entrained in the API or FDP as a production-related leachable. Clearly such an added entity is an impurity whose direct and indirect effect on the safety and efficacy of the API or FDP must be established. Although the regulations are clear that such interactions and their effects must be evaluated and quantified, there is limited regulatory guidance or guidelines on how to specifically address and assess additive interactions between the process solutions and their associated production equipment.
As the use of plastic components in production systems has grown, so too has the concern that such components may affect the quality, or suitability of use, of the API or FDP. As a result of these concerns, terminology and strategies for assessing the impact have been developed and proposed (1, 5⇓⇓⇓⇓⇓⇓⇓–13).
Risk Management And Safety Assessment
The use of risk management principles and concepts to address the potential product safety risk associated with leachables (and extractables as potential leachables) is a cornerstone of global regulatory and industry thinking on this topic. Industrial scientists and regulators agree that the concepts and principles of risk management have a definite strategic role in terms of designing, implementing and interpreting effective and efficient assessments of extractables and production-related leachables. Oversimplifying somewhat, it is well-established that risk management tools and principles can be used to define the nature and magnitude of assessment (including testing), where low risk situations require reduced assessment (testing) versus high risk situations. Thus, regulatory guidance for container/closure systems makes very clear reference to, and makes very extensive use of, risk assessment processes and procedures to define testing requirements that differ as a function of the perceived risk (for example, Table 1 in the U.S. Food and Drug Administration [FDA] Container Closure Guidance, reference 14, reproduced here as Table I).
Examples of Packaging Concerns for Common Classes of Drug Productsb
In essence, the FDA's Table 1 is a two-dimensional risk management matrix for use in the safety assessment of packaging systems. The dimensions in Table 1 that establish the degree of concern (risk) that a safety-impacting interaction will occur between a product and its packaging are the route of administration of the product and the likelihood that an impactful interaction will occur. Depending on where specific dosage forms fall in this matrix, the dosage forms are classified as having low to highest risk with respect to a safety-impacting interaction. Such a classification has a direct and clear impact on the type and magnitude of testing required to properly establish and manage the risk of any safety-impacting interactions that could occur between packaging and a packaged drug product.
Clearly there would be considerable value in the development of a similar risk assessment tool for production systems. Such a tool would establish the relative risk that is associated with a given production situation and based on that risk would indicate what level and type of assessment would be adequate to address the risk. Because of the complexity of the production process, it is clear that such a tool would have to consider multiple dimensions and would have to establish the aggregate impact produced by all the individual dimensions.
Such a tool, referred to in terms such as risk evaluation worksheet (15) and risk assessment matrix (16), has been proposed by numerous authors (1, 5, 10, 15⇓–17). Although the exact form and content of these devices differ somewhat among the various authors, the devices share a common theme, as illustrated in the generalized risk evaluation matrix shown in Table II. In such an approach, several contributors to risk are identified and used to construct the matrix. These contributors may include things such as
The nature of the material itself and its inherent “resistance to extraction” or “tendency to possess extractable substances”, or “tendency of the extractables to be toxic”,
The nature of the solution itself and its “extraction capability” or “leaching power”,
Conditions of contact such as duration, contact surface area, temperature of contact, and
Proximity of the contact event to the release of the finished drug product from the production process.
Generalized Risk Evaluation Matrixa
To assess the safety risk using the risk evaluation matrix, the specifics of the contact situation are weighted via a ranking scale. Thus, for example, the risk reflected in contact duration might be weighted as “less than 24 h = low = 1”, “greater than 24 h but 30 days or less = medium = 5” and “greater than 30 days = high = 10”. Similar weightings would be given for the other contributors to the total risk. The final individual risk contributions are added up (or multiplied) and a final risk score is obtained. The magnitude of the final risk score would dictate how much and what type of chemical testing would be performed, where the lower the risk score, the lesser the testing.
If it is possible to support the concept of the risk management matrix but to have misgivings about proposed risk management matrices themselves, then this is the position of this author. While a risk management matrix has the foundations of good science because (a) it is a process and (b) it involves a calculation, there is reason to be concerned with the potentially arbitrary and non-science-based aspects of the matrix. Questions about the matrix that are open to scientific debate include the following:
Are all the relevant dimensions captured in the matrix?
Are the risk values used in each dimension science-based? In most situations, the published risk factors appear to be intuitive, experience-based, or arbitrary.
Are all the dimensions properly weighted? In most situations the various dimensions are equally weighted in terms of their impact on safety.
Is the aggregate effect of the dimensions additive (which is the process used in most applications) or are the inter-relationships between dimensions more appropriately expressed by a more complicated function?
Are risk scores properly calibrated? For example, how has one determined that a risk score of 15 is “safe” and a risk score of 60 is “unsafe”?
What are the criteria or tests necessary to establish the qualitative “levels” within each risk variable? For example, how does one establish whether a material is “reactive”, “interactive”, or “inert”?
Given the great diversity of manufacturing operations, is it possible that a single matrix can be adopted that will “cover all the bases”?
It is this author's assertion that unless the specifics of risk evaluation matrices can be scientifically justified, the matrices are, at best, a qualitative tool that provides only a simplistic, general or approximate, and potentially erroneous answer to the complex question of what type and amount of testing is necessary to obtain a rigorous safety assessment of production systems. Furthermore, there are two additional issues with the way in which risk evaluation matrices are generally applied. Firstly, it is the author's experience that there are nearly as many different matrices as there are companies that have such matrices. This lack of a standardized approach at least opens up the possibility that two companies, assessing the same component in the same process, could come to two different decisions in terms of the required testing. Secondly, it is the author's further experience that the lowest level of testing commonly attached to a risk evaluation matrix is little or no testing. It is difficult for this author to support the concept that components could be used in production operations with little or no testing to support their suitability for use, no matter how low the risk is. Rather, this author maintains that there is a minimum or baseline amount of information required to support the use of a component in production and that the lowest level of testing is procurement of this baseline information by whatever means are appropriate.
Lastly, this author notes the definition of risk itself, which is expressed as:
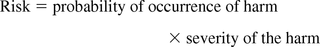
As defined previously, a risk evaluation matrix addresses only one term in this equation, the probability of the occurrence of harm. Thus by itself, the risk evaluation matrix does not allow for proper risk management. An effective risk assessment must address both the dimensions of probability and severity to be effective in matching the degree of required testing with the magnitude of risk.
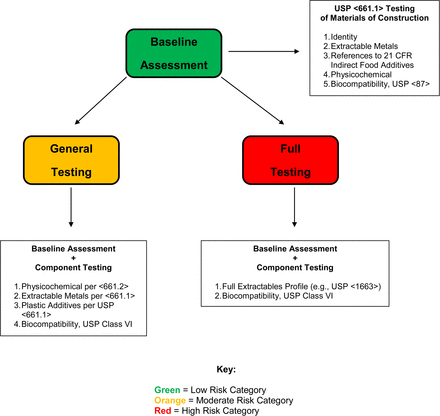
In this article the author proposes and justifies a risk evaluation matrix that guides users of plastic components in production suites in determining the type and amount of testing that is appropriate to select and qualify components. Furthermore, the paper establishes three “levels” of testing that link to the risk assessment matrix: baseline assessment, general testing, and full testing.
Generation and Justification of the Risk Evaluation Matrix
General
The proper use of any risk management strategy is based on a firm understanding of what risk is being managed. In this paper, the risk that is being managed is the risk that substances leached from a component used in the production suite would accumulate in the production process output (either an API or a FDP) at levels sufficiently high that they would adversely affect the health of a user administered the API or FPD. This risk is being managed based on information obtained by testing the component, or its materials of construction, as such chemical information reflects leachables behavior. The underlying principle of the risk evaluation matrix is that the amount and type of testing required to properly evaluate a component that is used in the production suite is related and relatable to the risk of such an adverse effect on user health. The higher the risk, the more comprehensive the testing would be. The risk evaluation matrix provides both a means of quantifying (or scoring) the risk associated with a particular circumstance and a risk scale that links the risk score to an appropriate testing study design.
Extractables from production components accumulate in the production process output because the production output (or a precursor solution) contacts and interacts with such components. These extractables accumulate in the production process output (API or FDP) as leachables only if they persist in the process stream through that last point in the production process where the process stream is fully converted to the production process output. Establishing a risk evaluation matrix that is relevant to production-related leachables involves five essential steps:
Identification of the factors of contact that contribute to the leachables risk,
Identification of the factors of production (and use) that mitigate (or exacerbate) the leachables risk,
Risk-scoring the factors individually,
Combining the scores for the individual factors into a composite or total risk score,
Comparing the score for a particular circumstance to a scale that links certain scores to certain testing actions.
Five Critical Factors
In considering the factors that must be captured in the risk evaluation matrix, one notes the following:
The conditions of contact between a production component and an API, FDP, or an associated precursor solution will have a direct effect on the magnitude of the interaction that will occur between the component and the API.
The properties of the contacting entities, both the component and the precursor solution, will have a direct effect on the magnitude of the interaction that will occur between the component and the solution.
What happens to precursor solutions in those stages of production that follow after the contact between the component and the precursor has occurred can either mitigate or exacerbate the magnitude of the interaction.
The nature (or dosage form) of the API or the FDP affects the patient exposure and thus the risk.
How the production processes' output (either the API or FDP) is used by the patient affects the patient exposure and thus the risk.
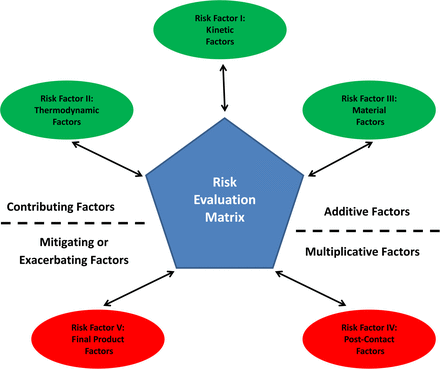
These observations led to the identification of five factors that together fully establish the risk that potentially unsafe production process–related leachables are present in the process output, API, or FDP (see Table III and Figure 1):
Risk Factor I (RFI): kinetic factor, considering those aspects of the contact that are kinetically driven.
Risk Factor II (RFII): thermodynamic factor, considering those aspects of contact that deal with the “leaching power” of the contact solution.
Risk Factor III (RFIII): material factor, considering those aspects of contact that are affected by the properties and processing of the contacted component and its materials of construction.
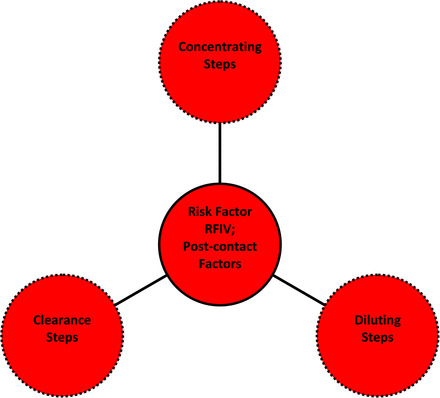
Risk Factor IV (RFIV): post-contact factor, considering aspects of the production process that occur after contact and that might mitigate or exacerbate extractables becoming leachables.
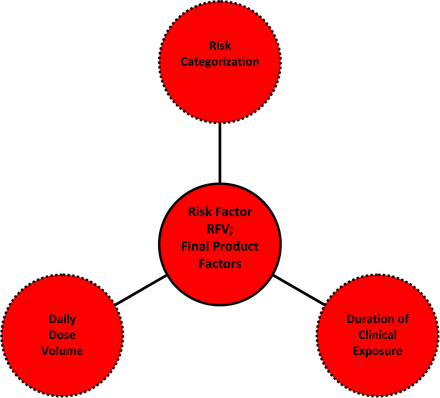
Risk Factor V (RFV): final product factor, considering aspects of the clinical use of the API (or FDP) that mitigate or exacerbate the effect of leachables on patient safety.
Risk Factors Used in the Risk Evaluation Matrix
The five risk factors (RFs) considered in the risk evaluation matrix. Three contributing factors address the kinetic, thermodynamic, and material-related facets of the actual contact between a process solution and a plastic production suite component. Two potentially mitigating (or exacerbating) factors address facets that are not related to the contact including (a) how the process stream is chemically modified between the point of contact with the plastic component and the final production of the drug product and (b) how the manufactured drug product is used in the clinical setting.
The framework of the risk evaluation matrix is defined by these five factors and how they are used to establish a total risk score. The framework of the risk evaluation matrix is constructed when (1) the individual aspects that are relevant to each factor have been delineated and a means of using the aspects to score each factor has been established, and (2) the means of combining the individual risk factor scores has been established and a total risk score can be calculated. The risk evaluation matrix becomes useful when the total risk score can be used to guide decisions on what testing is necessary to adequately address the risk.
The following sections of the paper present and discuss the considerations that went into the construction of the risk assessment matrix and ultimately justify the constructed risk evaluation matrix.
The Risk Assessment Model; Use of the Contributing Risk Factors To Calculate a Total Risk Score
The production-related extractables that have the potential to become leachables in a produced API or FDP are established when a component is contacted by the process solution. Thus the extractables profile that drives the safety risk is established by the interplay between those contact factors that define the magnitude of extraction, including Factors I through III noted previously. Because all these factors contribute to the extractables profile, it is proper that a risk score related to contact be calculated as the sum of the individual risk scores assigned to the individual factors. Once the extractables profile has been established, additional risk factors (IV and V) address the points that (a) the extractables' propensity to become leachables in the API or FDP may depend on the extractables persisting in the process stream as the stream makes its way through the production process (Factor IV), and (b) the extractables' effect on patient safety, once they are present in the drug product as leachables, will depend on how the drug product is administered (Factor V). These non-contact-related factors do not contribute to the extractables profile; rather, their effect is to mitigate or exacerbate the effect of the extractables as they become leachables. Thus the appropriate mathematical means of using these non-contact factors is as multipliers of the combined contact risk score.
Thus the appropriate means of calculating the total risk score (TRS) is captured in eq 1:
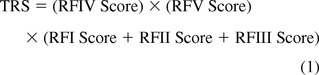
Contributing Factors Related to the Contact
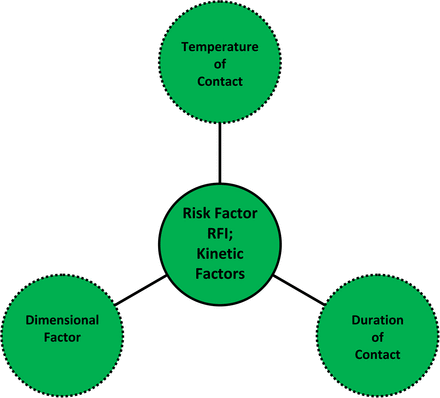
Risk Factor I (RFI): Kinetic Factor (See Table IV and Figure 2):
Factor I Analysis: Kinetic Factors (RFI Score)
The three aspects of the kinetic risk factor, RFI. The kinetic risk factor RFI considers the conditions of contact between a process solution and a plastic production component that influence the rate at which the interaction between the solution and the component occurs, including the temperature of contact, the duration of contact, and the dimensional aspects of the contact (such as component thickness and component surface area to solution volume ratio). Although dimensional factors are relevant to the interaction between a component and a process solution, the risk evaluation matrix does not include a rigorous assessment of this aspect.
The leaching of a material's constituent by a contacting solution requires the movement of the constituent through the material via diffusion. The diffusion of constituents through polymers has been extensively studied; conditions of contact that strongly influence the magnitude of migration (diffusion) include the contact time, the contact temperature (as temperature affects the diffusion coefficient), and certain dimensional aspects such as the length of the diffusion path (relatable to the thickness of the contacted material) and the contact surface area. These impactful conditions of contact thus establish those aspects that are germane to the kinetic risk factor, Risk Factor I (RFI).
Considering contact time, it is well-established that the magnitude of migration is proportional to the square root of migration time; see, for example, eq 2 (18).
 where Mt = amount leached into solution at time t, M∞ = equilibrium accumulation at infinite time, D = diffusion coefficient, and δ = the polymer's thickness.
where Mt = amount leached into solution at time t, M∞ = equilibrium accumulation at infinite time, D = diffusion coefficient, and δ = the polymer's thickness.
Considering temperature, the mathematical relationship between the diffusion coefficient and temperature is well-defined; however, obtaining relevant input values to quantify temperature effects, such as activation energies, may be challenging. Thus a more qualitative relationship between the diffusion coefficient (D) and contact temperature may be adequate for the purpose of risk assessment and management. In such a circumstance, a qualitative generalization, known as the factor 10 rule (19), may be applicable. This factor 10 rule is based on the observation that activation energies for migrating substances in polymers relevant to packaging are typically in the range of 80 to 100 kJ/mole. In such a circumstance, the diffusion coefficient increases by roughly an order of magnitude for every 20 °C increase in contact temperature. Thus for example, the migration rate at 40 °C is 10 times faster than the migration rate at 20 °C:
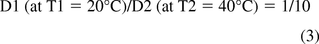
These observations concerning the effect of contact duration (time) and temperature on the magnitude of migration can be used as the mathematical basis for scoring Risk Factor I (RFI). The scoring is accomplished by defining a baseline contact time and temperature and assigning the baseline a reference RFI score. RFI scores for other times or temperatures are based on the difference between these times and temperatures and the baseline conditions and reflect adjustments to the reference RFI. The baseline contact conditions were established as room temperature (20 °C) for 1 day and were assigned a RFI score of 10. RFI scores at other times and temperatures are obtained by multiplying the reference RFI score by factors related to time and temperature:
 where tf = a time factor comparing t to 1 day and Tf = temperature factor comparing T to 20 °C. The values of tf and Tf are consistent with the generalizations noted previously, thus Tf reflects factor of 10 multiples for each 20 °C difference between t and the baseline value of 20 °C, and tf reflects the square root of the absolute difference in T versus 1 day. The square root used in tf reflects the relationship between migration amount and time per eq 2. These concepts are illustrated in Table IV, which shows RFI scores for specified time and temperature combinations. For example, consider the case of contact at 40 °C for 30 days, which may be an applicable acceleration of long-term ambient temperature storage of a process intermediate in a plastic bag. The RFI score for this situation is calculated as follows:
where tf = a time factor comparing t to 1 day and Tf = temperature factor comparing T to 20 °C. The values of tf and Tf are consistent with the generalizations noted previously, thus Tf reflects factor of 10 multiples for each 20 °C difference between t and the baseline value of 20 °C, and tf reflects the square root of the absolute difference in T versus 1 day. The square root used in tf reflects the relationship between migration amount and time per eq 2. These concepts are illustrated in Table IV, which shows RFI scores for specified time and temperature combinations. For example, consider the case of contact at 40 °C for 30 days, which may be an applicable acceleration of long-term ambient temperature storage of a process intermediate in a plastic bag. The RFI score for this situation is calculated as follows:
The Tf value is 10, as the difference between the reference temperature of 20 °C and the contact temperature of 40 °C is 20 °C.
The tf value is (30/1)1/2 = 5.48, reflecting the difference between the reference time of 1 day and the contact time of 30 days.
Using these values for tf and Tf and eq 2 produces the RFI for this set of conditions:
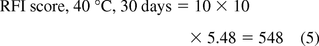
Although the potential values for the RFI score range from essentially 0 to a very large number, practically the range in RFI scores is limited by the conditions of contact experienced in typical production process scenarios. For example, under the conditions illustrated in Table IV, which represent reasonable approximations of extreme process conditions, RFI scores range from 1 to 1000. This range is significant with respect to ranges in scores used in other risk matrices, such as that presented in Table II. In the case of the risk matrix in Table II, the range in risk scores attributed to time and temperature would be from 2 to 20. This would suggest that the risk at the extreme highs in time and temperature is a factor of 10 greater than the risk at the extreme lows in time and temperature. In fact, the analysis performed in this paper suggests that the risk at the extreme highs in temperature and duration may be as much as a factor of a thousand greater than the risk at the extreme lows. Thus the risk matrix illustrated in Table II underestimates the increase in risk associated with extremes in time and temperature and could prescribe testing that is not adequate in terms of proper risk management.
As noted in Table III and eq 1, dimensional aspects such as material thickness and surface to volume ratio can also affect the magnitude of leaching. However, the effect of these dimensional aspects is more difficult to ascertain than is the effect of either temperature or time. For example, consider material thickness. As noted in eq 1, the amount of substance that has migrated from a material to a contacting solution depends on the length of the migration pathway, which is typically a material's thickness. The thinner the material, the quicker that migrating substances will accumulate in the contacting solution. While this effect can be significant, it is relevant only in those kinetically constrained contact conditions in which equilibrium is not achieved between the material and the contacting solution. Furthermore, while migration through a thinner material may be quicker, the magnitude of migration may be less because the total pool of an extractables will be smaller in a thinner film versus a thicker film. Moreover, this discussion, which essentially considers a monolayer material, becomes more complex in the case of multi-layered structures.
The aspect of surface area to solution volume ratio may be similarly complex. As was the case for thickness, the kinetic impact of surface area is realized only if the contact is such that equilibrium is not achieved between the contacting entities. Furthermore, the kinetic impact of the surface area to solution volume ratio depends on the nature of the migrating substance. If the migrating substance has a greater affinity for the plastic versus the contacting solution (the substance's plastic/solution partition coefficient is high), then the substance's concentration in the contacting solution is low and essentially constant when the surface area to solution volume ratio is varied. Only if the migrating substance is more soluble in the contacting solution than in the plastic (the substance's plastic/solution partition coefficient is low) will the substance's concentration in the contact solution vary as a function of the surface area/volume ratio.
These observations can be illustrated when one considers the definition of the plastic (p)/solvent (s) partition coefficient (Kp/s):
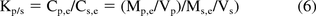 where C = concentration of the leachable in a particular phase, e means at equilibrium, i means initially (prior to interaction), M is the mass of the leachable in a particular phase, and V is the plastic (p) or solvent (s) volume. Equation 6 can be re-arranged so that the concentration of the leachable in the solvent at equilibrium is expressed as a function of the other quantities (for example, reference 19):
where C = concentration of the leachable in a particular phase, e means at equilibrium, i means initially (prior to interaction), M is the mass of the leachable in a particular phase, and V is the plastic (p) or solvent (s) volume. Equation 6 can be re-arranged so that the concentration of the leachable in the solvent at equilibrium is expressed as a function of the other quantities (for example, reference 19):
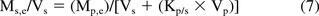
As noted, previously, when Kp/s is small (the leachable is highly soluble in the solvent), the term Kp/s × Vp is small versus Vs, and Ms,e is independent of Vs. When Kp/s is large (the leachable is highly soluble in the plastic), Vs is small versus the term Kp/s × Vp, and the concentration of the leachable in the solvent at equilibrium (Ms,e/Vs) is independent of Vs.
Thus, taking into account the dimensional aspects of thickness and surface area to solution volume ratio is complex and may require insights into extractables' properties, material properties, and application conditions that are not known at the time an extractables study is being designed and conducted. For this reason, a dimensional aspect is not included in the RFI score.
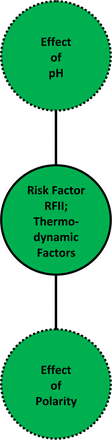
Risk Factor II (RFII): Thermodynamic Factor, “Leaching Power” of the Contact Solution (See Table V and Figure 3):
Factor II Analysis, Thermodynamic “Leaching Power” (RFII Score)
The two aspects of the thermodynamic risk factor, RFII. The thermodynamic risk factor RFII considers the properties of the process stream solution that establish it's “leaching power”, including the solution's pH and “polarity”.
In somewhat unscientific terms, when a material and solution phase come into contact and interact by leaching, the solution phase forces or drives the interaction to an extent dependent on properties of the solution while the material phase resists the interaction to an extent dependent on the properties of the material. Thus Risk Factor II (RFII) focuses on the “leaching power” of the contact solution while RFIII focuses on the “resistance” of the contacted material.
We start our discussion of the “leaching power” of the contact solution with the simplest such solution, water. Extractables present in the contacted material will accumulate in water as a contact solution to an extent established by the extractables' material/water partition coefficient, which can be approximated to some extent by the extractables' solubility in the contact solution. Thus when one considers how the composition of a contact solution affects its “leaching power”, one is actually considering what compositional factors of the contacting solution make it a better solvent than water. For ionic extractables, the pH of the contacting solution will have a well-known and marked effect on the extractables' solubility. For non-ionic extractables, the polarity of the contacting solution will have a marked effect on the extractables' solubility, where the polarity of the contacting solution is affected by the solution containing an organic solvent (for example, ethanol), the solution containing solubilizing agents (for example, polysorbate 80), or the solution being essentially a water-based suspension of a non-polar agent (for example, blood, lipid). Thus RFII considers and quantifies, to a certain extent, the effect of contact solution pH and polarity of the solubility of extractables in the contact material, thereby addressing the “leaching power” of the contact solution.
Addressing pH, this author has recently considered the effect that pH has on the solubility of typical ionic extractables, both acids and bases (20). Several key observations were made in this analysis:
The acid and base dissociation contacts (Ka and Kb) of ionic extractables generally fall in such a range that pH between 6 and 8 has little effect on a solution's “leaching power”.
The solubility of an acidic extractable increases as the solution pH is increased above (or higher than) its pKa, while the solubility of a basic extractable increases as the solution pH is decreased below (or lower than) its pKa.
For each pH unit above an acidic extractable's pKa (or below a basic extractable‘s pKa), the solubility increases by approximately a factor of 3. However, when the pH differs from the pKa by more than 2 units, the effect of pH on solubility is lessened and eventually solubility is pH-independent.
Empirically generalizing these observations, the risk that an extractables profile contains the largest number of ionic extractables at the highest concentration (1) is lower and essentially constant in a pH range between approximately 5.5 and 7.5, (2) increases by roughly a factor of 3 for each unit the pH is below 5.5 and above 7.5, and (3) at very low and very high pH values (pH < 1 or pH > 12), the effect of either increased or decreased pH on solubility is minimal (as few basic or acidic extractables have pKa values that are less than 2 or higher than 8, respectively). The score for the pH aspect of RFII, as shown in Table V, mirrors these trends. Thus the RFII score is “normalized” as 1 if the solution pH is between 5 and 8. At pH 5 and pH 8, the RFII score becomes 3 and increases by a factor of 3 for each unit increase or decrease in solution pH, producing a RFII score of 243 at pH 1 and pH 12 that is not increased further should the pH of the contacting solution be increased above 12 or decreased below 1.
Addressing polarity, this author has recently considered the effect that the proportion of ethanol in ethanol/water mixtures has on the leaching (solubility) of typical extractables (21). This analysis is relevant to contact solutions encountered in product suites, as ethanol is commonly used as a solvent in such contact solutions. This is also relevant to contact solutions that contain solubilizing agents such as polysorbate 80, albumin, and lipids, as the solubilizing power of such solutions can be related to model solvents that are simple ethanol/water mixtures.
In the referenced study, the proportion of ethanol in extracting solvents consisting of ethanol/water mixtures was linked to the amount of a target extractable, di-(2-ethylhexyl) phthalate (DEHP), that was extracted from a DEHP-plasticized poly (vinyl) chloride source material at equilibrium. The concentration of extracted DEHP is a reflection of the “leaching power” of the ethanol/water mixture and serves as the basis of the polarity aspect values listed in Table V. From this Table, one notes that while the polarity effect ascribed to ethanol/water mixtures is relatively small at ethanol levels of 30% or less, a 70% ethanol solution is 7600 times “stronger” as a extraction solvent than is water.
Three additional points were considered in evaluating RFII. The first point that was considered was the phenomenon of salting out. The phenomenon of salting out is well known in the analytical sciences as a means of facilitating the movement of solutes out of an aqueous phase and into an organic phase (expediting solvent switching) by increasing the ionic strength of the aqueous phase, Presumably, salting out would work in reverse if the extraction solvent had a sufficiently high ionic strength that potential extractables were salted out of the extraction solvent. Although contact solutions encountered in the production suite could have high salt contents (ionic strengths), the ionic strength is typically too low to have an appreciable effect on the accumulation of extractables in the extracting solvent. Furthermore, accounting for salting out in the RFII score would result in a reduced risk potential; thus not accounting for salting out is the worst-case approach. Lastly, it can be difficult to generalize and quantify the salting out effect. Thus salting out is not considered in the risk evaluation matrix proposed in this paper.
The second point was the use of pure solvents in the production suite. As this is somewhat rare, a means of establishing the “leaching power” of such solvents was not included in this paper, although presumably in these rare cases one could establish the polarity of the pure solvent and adjust the risk accordingly, as was done for ethanol/water mixtures and the polarity aspect factor. Lastly, the impact of pH on the “leaching power” was only considered in the context of the pH effect on the speciation of acids and bases. Although pH might affect the composition of an extract by means other than solubility, for example, the acid- or base-catalyzed degradation of an extractable, such an effect is not relevant for most extractables and is difficult to ascertain without knowledge of a material's extractables profile and thus was not captured in the calculation of the RFII score.
In closing out the RFII analysis, one notes that pH and polarity effects are considered independently. Although it is possible that contact solutions used in the production suite might contain both buffers and a solvent or solubilizing agent, buffered solutions generally have a low ethanol content (or low ethanol equivalent polarity), and thus the pH effect is predominant over the polarity effect. Should the contact solution have a higher ethanol content, the polarity effect would be predominant over the pH effect. In those relatively few cases where neither pH nor polarity is dominant, and the “leaching power” of the contact solution is a combination of both the pH and polarity influences, it is suggested that the value of the polarity aspect be the simple sum of the pH and polarity factors. Thus,
In the case of a 20% ethanol solution at a pH of 10, the pH effect would be dominant and the polarity aspect would have a value of 27 (linked to the pH).
In the case of a 50% ethanol solution at a pH of 5, the ethanol effect would be dominant and the polarity aspect would have a value of 450 (linked to the ethanol content).
In the case of a 20% ethanol solution at pH 8, neither effect is dominant and the polarity aspect would have a value of 7 (3 for the pH 8 and 4 for the 20% ethanol).
This range in RFII scores is significant with respect to ranges in scores used in other risk matrices, such as that presented in Table II. In the case of the risk matrix in Table II, the range in risk scores attributed to polarity would be from 1 to 10. This would suggest that the risk for highly non-polar solvents is a factor of 10 greater than the risk for water. Although the risk matrix in Table II does not take into account solution pH effects, it is not uncommon to find risk matrices that allow for another factor of from 1 to 10 to account for solvent pH. However, the analysis performed in this article suggests that the risk at the extremes in solvent pH and polarity are many times higher than just a “factor of 10”. Thus the risk matrix illustrated in Table II underestimates the increase in risk associated with extremes in contact solution pH and polarity and could prescribe testing that is not adequate in terms of proper risk management.
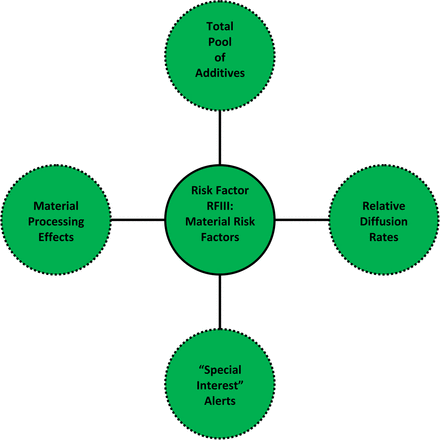
Risk Factor III (RFIII): Material Factors (See Table VI and Figure 4):
Factor III Analysis, Material Factors (RFIII Score)
The four aspects of the material risk factor, RFIII. The material risk factor RFIII considers the properties of the contacted material (or component or system as a set of materials) that establish its “resistance to leaching”, including its total pool of potential extractables, its permeability to extractables, whether the material has been subjected to processing steps that exacerbate extractables, and whether the material has been linked to “special case” extractables.
It is intuitive that the properties of a material of construction for a component or system in the production suite will strongly influence the risk associated with leachables from that material. This is typically captured, as shown in Table II, in one or two “factors”, the “chemical resistance” of the material and its composition. The general concepts here are that (1) a material that is resistant to extraction is lower risk, and (2) a material that contains fewer sources of extractables (that is, a material that is “cleaner” from an additives perspective) is lower risk. The issue faced in this article is to link the more nebulous characteristics of “resistant” and “clean” to more quantitative material properties and then to establish the relative impact that these properties have on the safety risk.
When considering the circumstance of chemical resistance, a shortcoming in some of the predicate risk matrices is that they equate chemical resistance to chemical compatibility. Thus, for example, users of the risk matrix would review a material's compatibility table (for example, a material is compatible with strong acids) and use this information to determine if the material was inert, reactive, or something in between. This practice is unsound because compatibility is frequently related to material properties (such as ability to withstand dissolution), which may have little to do with resistance to extraction.
A more appropriate material property that establishes its resistance to extraction is the material's diffusion coefficient. Materials with lower diffusion coefficients will be resistant to extraction, as the migration of the extracting solvent and the extracted substances through the material will be slow.
Relative diffusion coefficients for several plastic materials that may be used in the production suite are summarized in Table VII; as noted in that table, the diffusion coefficients can vary by as much as 5 to 6 orders of magnitude over the range of materials used in pharmaceutical applications. These diffusion coefficients are translated to diffusion rate (DR) aspect scores that range from 1 (plastic with D40 less than 1 × 10–13 cm2sec–1) to 100 (plastic with D40 greater than 1 x 10–8 cm2sec–1). The range in diffusion rate aspect scores is much smaller than the numerical range in D40 values as (a) the magnitude of migration depends on the square root of D and (b) the lesser importance of diffusion rate to the overall risk associated with extractables migration and accumulation.
Worst-Case Diffusion Coefficients at 40 °C (from Reference 22 Unless Noted)
Three additional material properties are captured in the RFIII score: (a) the level of total additives in the material, (b) whether the material is subjected to a thermal or radiation stress when it is used in a component or system of the production suite, and (c) the association of the material with “special interest” extractables. The assignment of material properties risk scores based on these three additional material properties is somewhat more arbitrary than in the case of other aspects, as there are no mathematical models or equations that link these material properties to a risk score. In the case of total additives, total additive levels range from trace constituents (<0.01% by weight, which are assigned a score of 1), to major components (>10% by weight, which are assigned a score of 500), with roughly a factor of 5 increase in risk score with every factor of 10 increase in the total additives amounts. The factor of 5 used to establish the risk score reflects the observation that the levels of extractables in an extracting solution will increase as the pool of the extractable (or its parent compound) in the material increases but that the magnitude of increase may be dependent on the extractable itself. As noted previously, the accumulation level for an extractable that is highly soluble in the extracting solution will be strongly dependent on the amount of the extractable (or its parent compound) in the material being extracted while the accumulation level of an extractable that is poorly soluble in the extracting solution will be much less dependent on the extractable's total pool.
The second property that is considered in the material property RFIII score is whether the material is processed prior to or during its disposition in a component or a system. The underlying principle here is that a stressed (or processed) material will have a more significant extractables profile than a corresponding unstressed material. This is the case because the stress causes additives in the material to degrade, with the degradation products increasing the number and amount of extractables. Furthermore, the degradation products associated with the stressed material are typically more soluble than the parent compounds.
Now it is the case that most plastic components used in the production suite have been formed into components via processes that involve a significant thermal and mechanical stress; for example, molding and extrusion. It is generally the case that subjecting a plastic material to the heat stress associated with typical processing steps (molding, extrusion, etc.) increases the number and levels of semi-volatile and non-volatile extractables (24), although the effect of heat on a material's extractables profile can depend on a number of factors (such as the material type and the material's initial composition) (25). Because this type of manufacturing-related stress is essentially a baseline for plastic components, manufacturing-related processing is not captured in the RFIII score. Rather, the RFIII score is based on whether the component is subjected to further processing prior to its use in the production suite. For example, it is not uncommon for components to be sterilized prior to use in the production suite, typically by exposure to either high temperature or ionizing radiation. Especially in the case of ionizing radiation, the effect of such processing on a plastic's extractables profile is profound and impactful (for example, references 26⇓⇓⇓⇓⇓⇓⇓–34). Thus RFIII contains an aspect that accounts for the increased risk associated with increased extractables profiles resulting from processing steps that involve material exposure to thermal or radiation stress. Somewhat arbitrarily, the additional factors associated with thermal and radiation stress are assigned scores of 50 and 200, respectively, while an un-processed material is assigned a score of 1.
The final aspect included in the RFIII score address the situation that certain extractables (leachables) have been identified as being of “special interest” or of being in a “special case”, meaning that such extractables have special safety or historical concerns that require that they be assessed more specifically than extractables in general (35). For example, polyaromatic hydrocarbons, N-nitrosamines, and 2-mercaptobenzothiazole have been established as “special case” leachables associated with rubber components used in packaging/delivery systems for orally inhaled and nasal drug products (OINDPs) (36). Additional extractables that might be considered to be of special interest include
Phthalates and Bisphenol A, given the amount of research that has been performed in regard to the potential safety impact of these chemicals.
Tungsten, given the documented ability of this metal to aggregate proteins (37⇓–39)
Bis(2,4-di-tert-butylphenyl)phosphate (bDtBPP), a degradation product of the antioxidant Irgafos 168, due to its association with reduced yields in growth media stored in plastic single-use bioprocess containers (34, 40) (and not due to any documented ability to adversely affect patient safety).
Cadmium, lead, arsenic, mercury, cobalt, vanadium, and nickel, as these elemental impurities have been established by ICH to be elements to be considered in all circumstances, regardless of whether or not the element is intentionally added to a drug product and regardless of the route of administration (41).
It is noted that this list of potential special case extractables is by no means definitive or complete, and it is doubtless the case that other special case extractables exist and could be recognized as such by an appropriate regulatory authority and/or standards-setting organization.
In any event, the known association of these “special case” extractables with a material of construction increases the risk that a component containing that material will leach the “special case” extractable under the production conditions of contact, thus increasing the risk that the extractable will accumulate as a leachable in the API or FDP. For such materials, this increased risk is accounted for in a score of 200 that is added to the RFIII score to address the alert aspect.
Considering all these aspects, the total RFIII score is the additive combination of the scores for each of its aspects (see Table VI):

The RFIII score can range from 3 (very slow diffusion, low additives levels, no processing and no alerts) to 1000 (very fast diffusion, high additives levels, exposure to ionizing radiation, special case alerts).
Post-Contact Mitigating (or Exacerbating) Factors:
Risk Factors RFI through RFIII all take into account the circumstances involved with the actual contact between a material (or component) and a solution present in the production suite. Because production is a process and not just a single event, there are events that could occur after contact to exacerbate or mitigate the risk that extractables derived from the contact will accumulate as leachables in the process output (API of FDP). Such events must therefore be taken in to account in the risk evaluation matrix. Furthermore, it is understood that the safety risk posed by a leachable in a FDP will depend greatly on how that FDP is administered to a patient. Thus, for example, the safety risk associated with a leachable in a FDP would be less if the FDP were a solid oral dosage form that was administered to the patient in an acute therapy as opposed to if the FDP were an aqueous injection, administered to the patient in a long-term therapy. Thus the circumstances of FDP use must be taken into account in the risk evaluation matrix.
Risk Factor IV (RFIV): Proximity of Contact to the Final Process Step (See Table VIII and Figure 5):
Factor IV Analysis, Process Factors (RFIV Score)
The three aspects of the process risk factor, RFIV. The process risk factor RFIV considers those actions that happen during the production process after the process stream solution has contacted a material or component, as such actions may affect the extractable's ability to persist through the production process and become entrained in the process output (drug product) as a leachable. RVIV considers three such actions, two of which could mitigate extractables becoming leachables (dilution or clearance process steps) and one of which could exacerbate extractables becoming leachables (concentration steps).
In the risk evaluation matrix shown in Table II, the possibility that an extractable would be affected by a production process step that occurs after the material/solution contact occurred is addressed in a very qualitative manner, essentially relying on the generalization that the further upstream the contact step is, the greater the likelihood that the extractable will be removed from, or diluted in, the process stream prior to the final process step that produces the process output (API or FDP). If there are no intervening steps between the contact step and the final processing step, then the extractable is most likely not removed or diluted in the final processing step and the risk of an extractable becoming a leachable is high. As the number of process steps between contact and final processing increases as one goes further upstream in the production process, there is a greater likelihood that one of the intervening steps will remove the extractable from the process stream, thus reducing (or eliminating) the risk of leachables accumulating in the process output.
It is clear that such an approach is deficient in at least three ways. Firstly, it is not the number of process steps that decreases the extractables risk, it is the type of steps that decreases the risk. In order for the extractables risk to be reduced, either the amount of the extractable in the process stream has to be reduced (for example by dilution or solution diversion) or the extractable has to be eliminated from the process stream (for example, by a process that is designed to purify the process stream). Furthermore, this approach ignores the possibility that a process step might concentrate the extractable, increasing the risk. Most importantly, however, the approach treats this factor as an additive factor, which is to say that the risk associated with intervening steps (or lack thereof) is added to the risk associated with the conditions of contact. This is not the correct approach because of the effect that the intervening processing steps has on the process stream that contains extractables. Because the intervening steps will either mitigate or exacerbate the risk of an extractable becoming a leachable, the correct way to use a proximity risk factor is a multiplier of the total contact-related risk factors. If the intervening steps are such that extractables are reduced or removed from the process stream, then the proximity risk factor would be assigned a value less than 1 and multiplied by the total contact-related risk factor. If the intervening steps are such that extractables are concentrated in the process stream, then the proximity risk factor would be assigned a value greater than 1 and the total risk would be the product of the proximity risk factor and the total contact risk factor.
Thus the process of proximity risk assessment becomes essentially an examination of the processing steps between contact and final output to ascertain what effect the steps could have, if any, on the levels of extractables in the process stream. Three types of processing steps are of particular interest:
Clearance steps, which are processing steps that have been confirmed to remove extractables from the process stream. For example, diafiltration is a process that is used to purify proteinaceous components in process streams. Diafiltration is a technique that uses ultrafiltration membranes to remove, replace, or lower the concentration of salts or solvents from solutions containing proteins, peptides, nucleic acids, and other biomolecules. The process selectively utilizes permeable (porous) membrane filters to separate the components of solutions and suspensions based on their molecular size. An ultrafiltration membrane retains molecules that are larger than the pores of the membrane while smaller molecules such as salts, solvents and water, which are highly permeable, pass through the membrane. Because extractables are typically small molecules, it is presumed that extractables would be separated from protein bulks via diafiltration, an effect that has been documented in the literature (42, 43). Another example is the pre-use filter integrity test step. Because the filter needs to be fully wetted prior to the integrity testing, the wetting fluid (either water or a water/alcohol mixture) flushes the filter and its associated components (tubing and connector). It is reasonable to expect that extractables associated with the flushed components would be removed from the process stream as the flushing solution is discarded.
Dilutive steps, which are processing steps in which the process stream solution containing the extractables are significantly diluted. For example, during the media and buffer filtration steps, extractables from filters, connectors, and tubing are diluted in large-volume bioreactors and pool vessels. As these process steps may involve large volumes on the order of hundreds to thousands of liters, extractables from the filters, connectors, and tubing are significantly reduced in concentration by these process steps.
Concentrative steps, which are processing steps which process stream containing the extractables is significantly concentrated (e.g., reduced in volume). For example, centrifugation and tangential-flow-filtration are often used during the harvest step to concentrate protein-containing solutions. These processes separate the protein of interest from process impurities including cell debris and lipids and produce a more concentrated protein fraction. Extractables that have some affinity for the protein or that behave like the protein will also be concentrated by these process steps.
Table VIII summarizes the means by which the proximity factor is addressed. Thus, if there are no dilutive, concentrative, or clearance steps between the contact step and final process step, then the RFIV score is 1. If there is a dilutive step in the process, the RFIV factor is reduced by a factor of 2, unless there is process data that suggest a greater reduction (lower factor) is achieved. If there is a clearance step in the process then the RFIV factor is reduced by a factor of ten, unless there is process data that suggest that a greater reduction (lower factor) is achieved. If there is a concentrator step in the process, then the RFIV factor is increased by a factor of 2, unless there is process data that suggest that a smaller factor is more appropriate.
Risk Factor V (RFV): Final Product Factors (See Table IX and Figure 6):
The three aspects of the final product risk factor, RFV. The product risk factor RFV notes that the risk being managed is not that the drug product will contain a production-related leachable but rather that that leachable will have an adverse effect on the safety of users of the drug product. Thus RFV considers those product factors that affect the safety effect of leachables, including the drug product's risk categorization, duration of clinical use, and daily dose volume.
Risk Factors I through IV are process-related and specifically address the accumulation of leachables in the process output (API or FDP). Although the safety risk related to leachables is clearly related to both the amount of leachables in the API or the FDP and chemical nature of leachables, the risk is also related to the way in which the patient is exposed to the leachable (that is, the form of the FDP and the way it is used in the clinical setting). Specifically, it is well established that route of administration and the physical nature of the FDP are both relevant in establishing the inherent safety risk of leachables; for example, Table I from the FDA container closure guidance. Furthermore, the duration of patent exposure is a significant factor to consider in establishing the safety risk to leachables, as in general the longer the clinical exposure, the greater the safety risk. Lastly, and for aqueous FDPs specifically, the daily dose volume, which in turn establishes the daily dose of a leachable, has a clear effect on the leachable's safety impact. The larger the daily dose volume, the greater is the daily exposure to, and potential safety impact of, a leachable.
These considerations were brought together as a fifth factor in the risk evaluation matrix. Considering the inherent product risk, the FDA Table I has been divided into four zones of varying risk; see Table IXA. The combination of route of administration and dosage form that has the highest risk (as specified by the FDA Table 1) is assigned a factor score of 10. Combinations of route of administration and dosage form that reflect a lesser but still considerable risk are assigned a factor score of 5. Combinations of route of administration and dosage form that reflect a still lower risk are assigned a factor score of 2. Combinations of route of administration and dosage form that reflect the lowest risk (but not zero risk) are assigned a factor score of 1. As the reader can surmise, these factor assignments are largely subjective and are not data-based.
Factor V Analysis, Product Factors (RFV Score), Inherent Product Riska,b
The duration of exposure risk is addressed by leveraging the concept behind the staged threshold of toxicological concern (TTC), which is applied the qualification of genotoxic and carcinogenic impurities in drug products (44). The staged TTC concept establishes that the longer the clinical exposure, the lower the acceptable safety qualification threshold for genotoxic and carcinogenic impurities, implying that the longer the clinical exposure, the greater the safety risk. Thus Table IXB leverages Table I from reference 44 and somewhat arbitrarily assigns a duration-of-exposure risk score to each one of the durations listed in Table I.
Factor V Analysis, Product Factors (RFV Score), Duration of Exposure Riska
The aspect of daily dose volume as a driver of safety risk is well-recognized in the extractables and leachables community, and the increased safety risk associated with high daily dose volumes, which translates to increasingly smaller analytical evaluation thresholds for leachables, has been termed the “LVP challenge” (45), consistent with the high daily dose volumes associated with large-volume parenterals (LVPs). This aspect is illustrated via the following example. Normal saline for injection, packaged in plastic containers, may be used as a diluent for drug product admixtures. A product unit of normal saline for injection will contain a certain level of a certain leachable. In this example, product units are used as the diluent for two admixed drug products with two different therapeutic applications with two different dosing regimens, one of which involves the administration of a single product unit and the other of which involves the administration of multiple product units. It is clear that the patient exposure to the leachable (and thus the corresponding safety risk that the leachable poses to the patient) is greater in the case where multiple product units are administered.
Table IXC establishes four categories with respect to daily dose volumes and assigns a daily dose volume risk score to each category. Although the range in daily dose volumes relevant for therapeutic products spans multiple orders of magnitude, the range in the daily dose volume risk score is only one order of magnitude as RFV captures all the aspects related to product use and it is inappropriate to overweight the effect of dose volume relative to the other aspects by allowing the dose volume score to increase in direct proportion to the daily dose volume.
Factor V Analysis, Product Factors (RFV Score), Daily Dose Volume Risk
As shown in Table IXD, the risk score associated with RFV is obtained as the sum of the risk scores associated with the three aspects of RFV:

Calculation of the Factor V Product Factors (RFV) Score
While the absolute value of the RFV score is important, the critical issue is how to translate the RFV score into a multiplier that can be applied to the contact risk score to either mitigate or exacerbate the risk. This issue was addressed by establishing that the RFV multiplier would have only four values: 0.1 for those situations where the product risk was so low that the contact-related risk is dramatically reduced, 0.5 for those situations where the product risk was sufficiently low that it would mitigate the contact-related safety risk, 1.0 for those situations where the product risk was such that it neither mitigates or exacerbates the contact-related risk, and 2.0 for those situations where the product use conditions exacerbate the contact-related safety risk. Linking RFV scores to the individual multipliers was accomplished by considering the entire universe of possible RFV scores and then logically correlating certain ranges in the RFV scores ranges to the four possible multipliers. This assessment is captured in Table IXE. The following logic was applied to produce the risk score to multiplier correlations:
Only those product use scenarios with the lowest risk should have a multiplier of 0.1. Considering the inherent product risk aspect, this meant that the combinations for route of administration and dosage form that gave inherent product risk scores of 5 and 10 could not be allowed to have a multiplier of 0.1. Furthermore, even in the case that the inherent product risk factors were lower (2 or 1), only low scores with respect to duration of exposure and daily dose volume would be justifiable in terms of supporting a multiplier of 0.1. This logic train established that the RFV scores would have to be 4 or less to justify a multiplier of 0.1.
Only those risk product use scenarios with the highest risk should have a multiplier of 2.0. Considering the inherent product risk aspect, this meant that the combinations for route of administration and dosage form that gave inherent product risk scores of 1 and 2 could not be allowed to have a multiplier of 2.0. Furthermore, even in the case that the inherent product risk factors were higher (5 or 10), only high scores with respect to duration of exposure and daily dose volume would be justifiable in terms of supporting a multiplier of 2.0. This logic train established that the RFV scores would have to be 25 or greater to justify a multiplier of 2.0.
Establishing the RFV scores that would differentiate between multipliers of 0.5 and 1.0 was based on the observation that most of the combinations of the three risk aspects should result in a multiplier of 1, meaning that the combined effect of aspects was to neither mitigate nor exacerbate the risk established by considering the contact risk. Such a distribution between situations justifying multipliers of 0.5 and 1 was obtained when the RFV score of 24 was set as the score that would differentiate between multiples of 0.5 and 1.0.
Possible Scenarios for the RFV Score
Use of the Total Risk Score to Establish the Appropriate Testing
As noted previously in eq 1, the total risk score (TRS) is calculated as the sum of the three contact factors (RFI, RFII, and RFIII) multiplied by the mitigating or exacerbating post-contact factors (RFIV and RFV); see Table X. Considering the minimum and maximum values that the various individual factors can possess, the possible range in TRSs is quite large, from a minimum value of 0.05 to a maximum value of 38,400. Qualitatively, this suggests that the patient safety risk posed by extractables that become leachables in the API or FDP is roughly 1 million times greater for the highest risk production situation than it is for lowest risk production situation, although this full range may not be encountered on typical production condition. This result is contrasted to the risk matrix shown as Table II, where the lowest score is 5, the highest score is 60, and the range in risk is approximately a factor of 10. As has been pointed out previously in this article, the difference in range for the risk evaluation matrix proposed herein versus other risk evaluation matrices is that the other risk evaluation matrices greatly underestimate the increased risk associated with more extreme and impactful process parameters. By underestimating the increased risk, such evaluation matrices could suggest insufficiently rigorous testing in high risk situations.
Total Risk Score and Categories
Ultimately the value of the risk evaluation matrix is not to obtain the TRS but to use the TRS as a means of establishing the level of testing that is necessary to select and qualify a component for use in a particular production operation. To accomplish this, the TRS must be calibrated in the sense that certain TRS values must be identified as transition points (that is, points where testing approaches are changed). Unfortunately, this author could not derive a master equation from scientific first principles that allows for the quantitative and science-based determination of these transition points. Rather, the author performed a situational analysis that involved calculating TRS values for many different scenarios. These scenarios and their resultant TRS values were reviewed in the context of the following question: “Under these circumstances, what is your expert, albeit semi-empirical, opinion on what level of testing should be required to select and qualify the component for use?”, where the possible answers were “baseline assessment” (lowest level of testing), “full testing” (extractables profiling, highest level of testing), and “general testing” (intermediate level of testing). Such a situational analysis was used to establish a low threshold, which is the cut-off TRS between baseline assessment and general testing, and a high threshold, which is the cut-off between general testing and full testing.
Exactly what comprises the various levels of testing will be considered later in this paper. At this time, the situational analyses that generated the low and high thresholds will be considered.
The situational analyses that are relevant to establishing the low threshold are contained in Table XI. Generally speaking, setting the low threshold involves providing answers to the question “under what circumstances is the risk so low that a baseline evaluation is sufficient to support the selection and qualification of a component?” Initially, this question was answered as follows:
Baseline testing assessment is appropriate in all situations in which the RFIV and RFV are both 0.1 (clearance step in the production process and low risk patient use), except for the most extreme contact conditions (for example, situations where the sum of RFI, RFII, and RFIII exceed 2500), and
Baseline testing is never appropriate by itself for situations where RFIV and RFV are both 2 (concentrator step and high risk patient use),
Conditions That Result in a TRS Score < 23, Thereby Allowing for a Baseline Evaluation. This List Does Not Include All the Possible Conditions That Would Produce a Baseline Evaluation Recommendation
In these circumstances, the low threshold would be the numerical values obtained when
TRS = 0.1 × 0.1 (2500) = 25 (corresponding to high possible values for RFI, RFII, and RFIII). This means that most low risk situations would have a TRS of 25 or lower, thus establishing that baseline assessment is the appropriate test approach.
TRS = 2 × 2 × (1 + 1 + 3) = 20 (lowest possible values for RFI, RFII, and RFIII). This means that the low threshold would have to be 20 or lower to meet the objective that baseline assessment is not an option for all high risk situations.
One observes from these results that the two criteria are in general agreement and that setting the low threshold at 23 will achieve the desired outcome. By assigning the low threshold a value of 23, the only high risk (RFIV and RFV both = 2) circumstances that would allow a baseline assessment are the cases where
The component's materials of construction have less than 0.01% by weight total additives.
The component's material of construction have diffusion coefficients of 5 × 10–14 cm2/s or less (very slow diffusion).
The component's materials of construction are not processed and are not linked to special interest extractables.
The conditions of contact are either 5 °C for 1 day (or less) or –20 °C (or fewer) for 100 days (or fewer).
It is likely the case that very few production situations would fit these criteria.
On the other hand, the following considers the implications of a low threshold of 23 for low risk (RFIV and RFV both = 0.1) circumstances.
All situations involving aqueous contact solutions (at all pH values) and contact solutions with 40% ethanol or less would require only a baseline evaluation.
All situations involving a contact solution that contains 70% or greater ethanol require additional testing to augment the baseline assessment.
The testing strategy for contact solutions between 40% and 70% ethanol would be established on a case-by-case basis and might or might not establish baseline evaluation as the only required testing.
Lastly, the following considers the “nominal” case where RFIV and RFV are both assigned a value of 1 (no special case processing steps and nominal risk patient use):
All situations involving aqueous contact solutions with a pH of 10 (or higher) or 3 (or lower) would require additional testing to augment baseline assessment.
All situations involving a contact solution that is 40% ethanol or greater would require additional testing to augment baseline assessment.
The status of contact solutions of 30% or less ethanol or between pH of 3 to 10 would be established on a case-by-case basis and might or might not establish baseline evaluation as the only required testing.
Focusing on the high threshold, the situational analyses that are relevant to establishing the high threshold are contained in Table XII. Generally speaking, setting the high threshold involves providing answers to the question “under what circumstances is the risk so high that a full testing is required to support the selection and qualification of a component?” Initially, this question was answered as follows:
All situations in which the RFIV and RFV are both 0.1 (clearance step in the production process and low risk patient use) should never require full testing.
For situations where RFIV and RFV are both 2 (concentrator step and high risk patient use), full testing should generally be required. Specifically, only in the circumstances where the sum of risk factors RFI, RFII, and RFIII is low, say a value of 25, would full testing not be required.
Conditions That Result in a TRS Score > 100, Thereby Triggering Full Testing. This List Does Not Include All the Possible Conditions That Would Produce a Full Testing Outcome
In these circumstances, the high threshold would be the numerical values obtained when
TRS = 0.1 × 0.1 (7600 + 1000 + 1000) = 96 (highest possible values for RFI, RFII, and RFIII). This means that the high threshold would have to be 96 or higher to meet the objective that all low risk situations never require full testing.
TRS = 2 × 2 × (25) = 100. This means that the high threshold would have to be 100 or higher to meet the objective that full testing is the proper option for all high risk situations except those with the most benign contact conditions.
One observes from these results that the two criteria are in general agreement and that setting the high threshold at 100 will achieve the desired outcome. Specifically, setting the high threshold at 100 means that no low risk (RFIV and RV both = 0.1) situation will require full testing. Additionally, the following considers the implications of a high threshold of 100 for high risk (RFIV and RFV both = 2) circumstances:
All situations involving aqueous contact solutions with a pH of 10 (or higher) or 3 (or lower) would require full testing.
All situations involving a contact solution that is 40% ethanol or greater would require full testing.
The status of contact solutions of 30% or less ethanol or between pH of 3 and 10 will be established on a case-by-case basis and might or might not require full testing.
Lastly, the following considers the “nominal” case where RFIV and RFV are both assigned a value of 1 (no special processing steps and nominal risk patient use):
All situations involving aqueous contact solutions with a pH of 12 (or higher) or 1 (or lower) would require full testing.
All situations involving a contact solution that is 50% ethanol or greater would require full testing.
The status of contact solutions containing less than 50% or less ethanol or between pH of 1 and 12 will be established on a case-by-case basis and might or might not require full testing.
A general distribution of testing requirements for various contact situations is contained in Table XIII. This general distribution of outcomes is consistent with the subjective expectations which were discussed previously and are consistent with the expectation that higher risk triggers more rigorous testing.
General Distribution of Contact Situations with Respect to the Required Level of Testing
The Three Levels of Extractables Testing: Baseline Assessment, General Testing, and Full Testing
The spectrum of TRS scores has been separated into three categories based on the situational analysis of all potential TRS values. The intent of this exercise was to establish three testing processes, each one associated with a specific category and each one consistent, in magnitude and rigor, to the degree of risk associated with the individual categories (see Figure 7). The concept inherent to this overall testing strategy is that a group of tests, the baseline assessment, performed on a component's materials of construction is the foundational requirement for all risk categories and the sole requirement for the low risk category. As the risk increases, baseline assessment is augmented by increasingly rigorous testing performed on the component. These sets of increasingly rigorous tests are termed general testing, applied to moderate risk situations, and full testing, applied to high risk situations. These three testing strategies are considered in greater detail as follows.
Diagram showing the testing required for a particular risk category. The baseline assessment is required for all risk categories and is the sole requirement for the low risk category. The testing specified for moderate risk (general testing) and high risk categories (full testing) augments the baseline assessment by requiring more comprehensive testing.
Baseline Assessment:
A component used in a production system consists of one or more materials of construction. For plastic components, the individual materials of construction are typically resins of a type of plastic. Some components consist of only one material, such as a polypropylene connector, silicone tubing, or a polyisoprene gasket. Other components consist of multiple materials of construction, for example, a multi-layered bag used as a bioprocessing reactor. In any event, it is reasonable to expect that a component's materials of construction were selected based on the characteristics and properties of the materials, and the choice of a particular material for a particular component can be justified based on those characteristics and properties. When the selection process considers the material's potential to contribute leachables to an API or an FDP, the characteristics of a material that is the basis of its selection include its composition and extractables behavior. Such an expectation is the basis of the USP testing paradigm for materials used in plastic packaging systems, as captured in its <661.1> monograph, Plastic Materials of Construction (46). For manufacturing systems, such an expectation is captured in the baseline assessment, where the baseline assessment is a series of tests, with associated acceptance criteria, that provide the compositional information that is the necessary basis for material selection and justification. Although the circumstances involved with packaging systems may differ from the circumstances involved with components used in the production suite, the essential principles of the USP packaging monograph are equally applicable to packaging systems and production components. Thus the baseline assessment for plastic components is modeled after <661.1> as follows:
All materials of construction used in a plastic component present in a production suite must have been characterized per USP Monograph <661.1> and must meet the specifications contained in that Monograph.
More specifically, the USP <661.1> Monograph provides tests and specifications that differ somewhat depending on the dosage form, as <661.1> is based on the risk-based approach that notes that the nature and magnitude of testing should be consistent with the risk. Because the baseline assessment is reserved for those production situations where the potential risk to patient safety is lowest, it is appropriate that the baseline assessment adopt the USP <661.1> tests and specifications meant for the lowest risk dosage forms. Thus the baseline assessment requires that all materials of construction
have their identities established,
are tested for extractable metals,
are established to be biocompatible by performing USP Biological Reactivity Tests, In Vitro <87>,
are tested for specified physicochemcial properties, and
are supported by appropriate reference to the indirect food additive regulations 21CFR 174-186, specifically addressing the purity criteria and limitations pertaining to use that are contained therein.
Full Testing, Extractables Profiling:
As the risk associated with a particular component increases, the nature of the testing required to qualify the component changes, typically requiring testing that is more directly indicative of suitability for use. It is appropriate that the testing for higher risk situations builds on the foundation established by testing performed for lower risk situations. Thus the materials of construction used in components that fall in the full testing category must meet the same requirements that are established in the baseline assessment. Additionally, the component itself must be tested to address the increased risk associated with situations that lead to the conclusion that full testing is required. In establishing the nature of this component testing, reference is made to the USP Monograph relevant for plastic packaging systems, <661.2> Plastic Packaging Systems for Pharmaceutical Use (47). Although <661.2> specifically targets packaging systems, its scope includes component testing.
In examining <661.2>, one notes that packaging systems (or components thereof) are tested as follows:
Biological reactivity per USP <87> and <88> as appropriate
Physicochemical tests (water extracts)
Chemical safety assessment (extractables profiling, leachables profiling, and associated toxicological safety assessment
As was noted previously, although the circumstances involved with packaging systems may differ from the circumstances involved with components used in the production suite, the essential principles of the USP packaging monograph are equally applicable to packaging systems and production components. Thus the full testing required for plastic components used in a production suite are the same tests that are required for plastic packaging systems in <661.2>.
It is important to note that while <661.2> requires extractables profiling, leachables profiling, when necessary and appropriate, and toxicological safety assessment of extractables and leachables data, it does not specify the test conditions and test methods that are to be used in the extractables or leachables studies. Recognizing that such conditions and methods would vary widely based on dosage form, <661.2> does not provide standard extraction conditions but rather directs users to USP Monograph <1663> Assessment of Extractables Associated with Pharmaceutical Packaging Delivery Systems (48), which provides the users with those scientific principles that they might consider in establishing the extraction conditions that are appropriate for their situation. It is this flexibility in approach that allows <661.2> to be equally applicable to packaging systems and production components. While the requirement to generate and safety assess an extractables profile is equally relevant to packaging systems and production components, the means by which the profile is generated and safety assessed might differ greatly for a packaging system and a production component. Although the means may be different, the scientific principles used to establish and justify the means are consistent across packaging systems and production and thus the knowledge and information contained in <1663> is applicable to both packaging systems and production components.
While the flexibility of <661.2> is a virtue to some members of the extractables/leachables community, it is a curse to other members of the community who seek standardized approaches. Debating the issue of flexibility versus standardization is outside the scope of this paper; however, for the sake of completeness this author notes that standardized extraction and testing methods have been proposed for single-use components (13). Please note that this citation is not to be construed as an endorsement or a censure by this author but merely an acknowledgement that such a proposal exists.
The safety assessment based on chemical testing is augmented by the orthogonal process of biocompatibility testing, which essentially requires that a component used in a high risk production situation must comply with the specifications relevant to a Class VI designation per USP <1031> The Biocompatibility of Materials Used in Drug Containers (49).
General Testing:
The general consensus among vendors, users, and regulators of plastic production components (and packaging systems) that high risk situations require the rigorous and extensive testing and analysis that is characteristic of extractables profiling, leachables profiling (as necessary and appropriate), and toxicological safety assessment—and that low risk situations require less rigorous and extensive testing—reflects the baseline assessment and full testing approaches discussed previously. However, the risk evaluation matrix was constructed to define a third and intermediate level of risk and thus this article is tasked with suggesting that testing that is appropriate to address those situations with intermediate risk.
In considering this task, it is clear that the requirements around materials of construction, which were the foundation of both baseline assessment and full testing, are also the foundation of the general testing. As was the case with full testing, general testing builds on this foundation by testing the component itself. The challenge in developing the general testing approach is to identify standard test methods that are less extensive than extractables/leachables profiling but that provide the required level of risk management. Two <661.2> tests which are already part of full testing, physiochemical tests and biological reactivity, fit such criteria and thus are adopted into general testing. However, these tests alone are not sufficient to address intermediate risk and thus they must be either fortified or augmented. In this vein, general testing is established to be:
Baseline assessment required for materials of construction,
Component testing for physicochemical properties per USP <661.2>,
Component testing for extractable metals by methods consistent with <661.1>,
Component testing for plastic additives, consistent with <661.1>, and
Component testing for biological reactivity sufficient that the component can be classified as USP Class VI.
Progression of Testing:
In examining the three levels of testing, one notes a progression of testing in two aspects: extractables and biocompatibility. All three levels of testing address these two aspects, but the means by which they address these aspects is correlated to risk, with the higher risk situations requiring more extensive testing. Thus in baseline assessment, extractables testing is reflected in physicochemical tests, extractable metals tests, and, for organic extractables, by proper reference to indirect food additive regulations. Biocompatibility testing is reflected in USP <87>. In the higher risk general testing, extractables testing is modified as the reference to indirect food additives required in the baseline assessment is replaced with specific tests for plastic additives. Considering biocompatibility, the USP <87> reference in the baseline assessment is replaced with the more rigorous compliance to USP Class VI designation. Lastly, in the highest risk cases associated with full testing, extractables testing is directly addressed by rigorous extraction and, as appropriate, migration studies, as opposed to being tangentially addressed on the basis of a component's composition (as revealed by its plastic additives). Considering biocompatibility, full and general testing both leverage the Class VI designation.
Conclusion
The use of risk evaluation tools to establish the level of testing that is necessary to select and qualify plastic components used in production suites for APIs and FDPs is a logical and appropriate extension of the use of risk-based approaches to ensure the quality, safety, efficacy, and stability of such pharmaceutical substances. A risk evaluation matrix can be derived and justified based on sound scientific principles and can be used to drive and define the chemical and biological testing that is appropriate to establish that plastic components in the production suite do no compromise the safety of APIs or FDPs produced by that suite. Application of such a matrix, and use of the testing strategies that are linked to the matrix, ensure that adequate, but not excessive, testing is performed.
Clearly, the application of the risk evaluation matrix contained in this article requires profound and extensive situation knowledge and involves a more or less involved series of calculations. It is possible that the purpose of the matrix, which is to align risk with the appropriate level of testing, could be achieved via a matrix that was simpler in its construction and use. However such a simplified matrix would be purposefully constructed consistent with the insights gained in the generation of the matrix contained in this article and it is reasonable to expect that the specifics of the simplified matrix would be linked to and justifiable by the scientific principles that are the basis of the matrix contained in this article.
Conflict of Interest Declaration
The author declares that he has no competing interests.
- © PDA, Inc. 2015