Abstract
The terminal sterilization of drugs and devices is the most appropriate means of assuring patient safety in terms of infection prevention. Adoption of terminal sterilization processes requires a supporting and thorough program for control and monitoring of bioburden, especially if a parametric release program of sterilization is desired. Such a control program should necessarily assess and evaluate the associated bioburden (primarily spores), which may resist inactivation and challenge the sterilization cycle. The bioburden resistance can be evaluated by several means and procedures (e.g., the boil test); however, these procedures should be designed and implemented taking into consideration the nature of the spore and spore recovery. This short review describes the application of moist heat resistance for the terminal sterilization of drugs.
LAY ABSTRACT: The terminal sterilization of drugs and devices is the most appropriate means of assuring patient safety in terms of infection prevention. Adoption of terminal sterilization processes requires a supporting and thorough program for control and monitoring of bioburden, especially if a parametric release program of sterilization is desired. This short review describes the application of moist heat resistance for the terminal sterilization of drugs.
- Microbiological tests
- Boil test
- Moist heat sterilization
- Terminal sterilization
- Overkill
- Bioburden
- Biological indicator
- Spores
- Spore inactivation
- Spore germination
- Spore outgrowth
- Bioburden control
- Parametric release
Introduction
Microorganisms are ubiquitous in the contributing elements of sterile product manufacturing, namely, components, raw materials, equipment, personnel, and pharmaceutical and medical device manufacture environment. ISO 11737-1:2006 describes bioburden as “the sum of the microbial contributions from a number of sources, including raw materials, manufacturing of components, assembly processes, manufacturing environment, assembly/manufacturing aids (e.g., compressed gases, water, lubricants), cleaning processes, and packaging of finished product” (1). Pre-sterilization bioburden is always present, and control over it is necessary to assure that the final product conforms to the requisite finished product quality sterility criterion. To this end, bioburden control is mandated through 21 CFR Part 211.110, Production and Process Controls, which states that “written procedures shall be established and followed that describe the in-process controls, and tests, or examinations to be conducted on appropriate samples of in-process materials of each batch” (2). Bioburden control is specifically listed as a requirement to assure the integrity of drug products. Bioburden control during the manufacture of sterile products is as much about knowing when to control as how to control (3).
In the manufacture of terminally sterilized products, validated sterilization processes impart the microbial quality attribute of sterility upon the finished product. These lethal sterilization processes may be designed, developed, qualified, and routinely controlled on the basis of the bioburden on or in the material. Given the diversity of bioburden (i.e., quantity, physiological state, physical state, resistance), coupled with the technical constraints of sampling and analysis, bioburden control is one of the greatest challenges in the manufacture of terminally sterilized products. Moreover, the determination of bioburden resistance is especially deserving of careful consideration, permitting the optimum monitoring and control strategy for effective, efficient, and compliant manufacture. Whereas radiation sterilization manufacture compliant with ISO 11137-1:2015 uses a thorough assessment of bioburden during cycle development, qualification, and periodic monitoring, bioburden-dependent moist heat sterilization processes require routine testing of the bioburden to quantitatively enumerate and evaluate potential resistance (4). Recent technologies, such as next-generation sequence analysis or flow cytometry coupled with single-cell sorting and bioinformatics, have led to profound progress in understanding the processes of spore inactivation (5). Thus, we briefly present options and requirements consistent with United States Pharmacopeia (USP) <1229> (6) and <1229.2> (7) for the moist heat resistance determination of microorganisms as part of an overall bioburden and process control strategy for terminally sterilized products.
Moist Heat Sterilization and Microbiological Resistance
The objective of lethal sterilization processes is killing microorganisms by exposing them to extreme physical or chemical conditions. Sterilization processes must balance competing and unrelated objectives. First, the process conditions must be sufficiently robust to reliably assure the destruction of the bioburden population present, thus fulfilling the microbial quality attribute of sterility; second, the conditions used must not be so harsh as to adversely affect the physical and chemical quality attributes of the materials being sterilized (6, 8). These material quality attributes include the functionally active formulation and the container closure system, which ensure sterility preservation. The selection of a specific sterilization cycle and its development must incorporate careful consideration of these competing objectives.
Moist heat sterilization cycles developed and qualified for sterile items commonly use biological indicators (BI) to confirm that sufficiently lethal conditions have been achieved per USP <1229> (6). The choice of an appropriate BI is guided by USP <1229.2> and is purposed as a surrogate representative spore derived from an appropriate microorganism (7). Moist heat resistance characteristics are one of the primary parameters used to select a BI, and resistance is typically required to be greater than the expected resistance of the encountered bioburden—a worst-case scenario. Moist heat resistance is described as the DT-value, which is the time in minutes required for a one-logarithmic (or 90%) reduction in the population of the BI under specified conditions (usually 121°C, i.e., D121°C). When material properties are not meaningfully affected by the sterilizing conditions, complete destruction of a large BI population with high moist heat resistance (worst-case parameters) during qualification is typically used to confirm process effectiveness. This is the common expectation when items to be sterilized are made of stainless steel, aluminum, or glass; this is referred to as the overkill validation method. Where material properties or other criteria require, a moist heat sterilization cycle may adopt a qualification and control process that is not based upon overkill but rather considers the moist heat resistance of the BI together with the anticipated population and resistance of the encountered bioburden. Doing otherwise can adversely affect material quality and preclude the use of a terminal sterilization process.
In 2013, the USP published the first chapters marking the start of a major revision in its sterilization-related content. This effort encouraged increased adoption of terminal sterilization processes, unequivocally benefiting patient safety. It was recognized that complete destruction of a large population of highly resistant BI (worst-case parameters, overkill sterilization) as the arbiter of cycle appropriateness was hampering the broader adoption of terminal sterilization processing (9). Accordingly, alternative methods of moist heat sterilization cycle design and qualification were described; these include bioburden/BI and bioburden methods to foster wider adoption of terminal sterilization processes.
This concept and the associated challenges are most evident when interpreting the desired probability of a nonsterile unit (PNSU) in relation to the BI population and resistance (see eq 1) (7), where the PNSU is the design end point of a sterilization process and is expected to be ≤10−6.
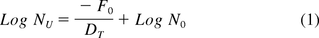 where Nu is PNSU, DT is DT-value of the natural bioburden, F0 is F0-value of the process (lethality), and N0 is Bioburden population per container.
where Nu is PNSU, DT is DT-value of the natural bioburden, F0 is F0-value of the process (lethality), and N0 is Bioburden population per container.
Calculating the PNSU using the DT value (moist heat resistance) of a BI ordinarily increases, to the detriment of the formulated product, both the cycle dwell time and the set point temperature. Employing this equation using the DT value for the anticipated resistance of the encountered bioburden is not straightforward. Although the bioburden population is easily determined, there are no widely published data sets providing moist heat resistance of typical bioburden microorganisms (10). The PNSU calculation (see eq 1) was added to USP's information chapter on sterilization for the following reasons:
The prevalent belief that killing the BI defined the PNSU was erroneous.
Sterilization cycle dwell time should not be based on the variable resistance of the BI (6).
Extreme assumptions about the required lethality estimated by the extreme thermal resistance of the BI have deleterious and easily avoidable effects on virtually all moist heat-sterilized materials (7).
The increasing usage of low-temperature moist heat for terminal sterilization was inhibited by PNSU expectations on the basis of BI resistance (11).
Adoption of sterilization processes qualified using the bioburden/BI or bioburden methods therefore requires a robust routine means of effectively monitoring both the population and resistance of bioburden.
Biological Indicator Evaluation Resistometer
The accurate determination of moist heat resistance requires equipment that is specifically designed for that purpose and conforms to ISO 18472:2006 (12). The Biological Indicator Evaluation Resistometer (BIER) vessel required for the moist heat resistance test is typically a specialized unit designed solely for the purpose and primarily for BI manufacturers, contract laboratories, and firms that use terminal sterilization extensively. Broader adoption of the bioburden/BI and bioburden methods requires an increasing need for frequent evaluation of the moist heat resistance of bioburden (assuring continued attainment of the requisite PNSU; see eq 1). This introduces the following significant practical difficulties, and considerations when using a steam BIER vessel for this purpose:
The steam BIER vessel's minimum operating temperature of 110°C specified in ISO 18472:2006 can be excessive for non–heat-resistant microorganisms (12).
Most BIER systems are single-purpose units, which can make them prohibitively expensive for occasional use.
Because bioburden resistance is not a standardized test in any pharmacopeia, users have adopted other methods to monitor their operations. The prevailing test is called the “boil test,” and while it has a singular name, its application varies substantially across the industry.
The Boil Test
The genesis of the boil test is ancient. References of boiling to treat drinking water first appeared around 2000 BC. The objective was to purify the water and prevent ill health. Antonie van Leeuwenhoek was the first to observe microorganisms in water in the late 1600s. In the mid-1800s, water treatment was first introduced to improve public health. It was later determined that boiling water for 1 min is sufficient to eliminate most common pathogens (13). Thus, globally, water is boiled daily and in the first world, periodically when citizens are instructed to boil drinking water under emergency circumstances. Boiling of water or the “boil alert” is not becoming obsolete, as it is one of the easiest methods to eliminate water-borne disease–causing microorganisms. In the context of pharmaceutical and medical device sterilization, the boil test relies on the same principles and can be used for numerous purposes in bioburden control and monitoring strategies. A primary healthcare application of the boil test is to distinguish moist heat–resistant microorganisms (primarily spores), which may pose a particular microbiological risk to product manufacture from non-resistant vegetative microorganisms. The test requires that a sample or item under evaluation is uniformly subjected to a temperature of 100°C for a specified duration, ensuring a constant thermal challenge over time. Any surviving viable microorganisms are then recovered by culture-based methods, permitting their enumeration and isolation for further analysis depending upon the purposed application of the test.
The boil test relies upon the fundamental observation that spores generated from differentiating microorganisms will retain their viability, while vegetative cells swiftly lose viability at 100°C. This is a useful generalization, as Bacillus vegetative cell inactivation with a decimal reduction time of 10 min has been reported to occur in the range of 40°C to 72°C. In contrast, Bacillus and Clostridium spores exhibit a decimal reduction time of 10 min in the range of 75°C to 121°C (14). The reader is directed to Leggett et al. for a useful review of the bacterial spore structural characteristics and the contributions each makes to spore resistance (15). These spore characteristics are important and deserve consideration in any terminal sterilization program; however, they are especially pertinent in the application of the boil test.
The boil test can be considered as two critical steps with two specific intended purposes. For the boil test to be effective, spores and vegetative cells must successfully show several activities at each stage of the test as illustrated in Table I.
Steps of the Boil Test and Required Microbiological Responses
Although a seemingly rudimentary test, the boil test is dependent upon spore physical and chemical characteristics and an often misunderstood and remarkably complex physiology of sporulation and germination. The boil test is influenced and affected by numerous factors that need to be considered with a judicious choice of test controls and parameters.
Inactivation
Moist heat treatment progressively damages several spore core and inner membrane proteins. Eventually, the spore's inner membrane ruptures, leading to dipicolinic acid (DPA), protein release, and rapid hydration of the spore. Subsequently, increased water content of the core further accelerates moist heat inactivation and irreparable damage of the spore core (16). The mechanism of inactivation and efficacy of moist heat inactivation during the boil test are acutely influenced by the genetics of the spore and the environment encountered as described by Warth (14). In addition to the hereditary genetic contribution, the prevailing environmental conditions (including but not limited to temperature and cationic elements) experienced during and immediately after sporulation influence a spore's moist heat resistance (17). These are important considerations when designing, controlling, and executing the step in the boil test purposed for inactivation of microorganisms possessing limited moist heat resistance.
Recovery
The recovery conditions (culture medium and incubation temperature and time) during the boil test must be carefully designed, controlled, and executed. This step allows surviving spores to (1) germinate, (2) support the outgrowth of germinated spores, and (3) concomitantly support replication to a discrete, visible colony-forming unit on solid media. These three physiologically distinct activities (germination, outgrowth, and replication) are performed by different biochemical entities, which vary depending upon species (18). Furthermore, the conditions in this step must be designed to allow spores that have been sublethally injured in their germination, outgrowth, or replication machinery to repair. The recovery and isolation step of the boil test is likely the most critical part of the test; insufficient recovery of a sporeformer with a D121-value of 1.9 min owing to the medium failing to support germination has been reported (19).
Presence/Absence of Spores
The most elementary application of the boil test is in the screening of materials for the presence of spores and spore-forming microorganisms. Although usually performed in an aqueous system (for example, a pre-sterilization intravenous solution), this screening permits rapid presence/absence confirmation that may be applied across the spectrum of sterilization modalities (e.g., dry heat, gas, and liquid sterilization processes) (20).
Screening for Moist Heat Resistance
Application of a boil test as part of a bioburden control and monitoring program supporting the manufacture of terminally sterilized products represents significant value if performed appropriately. For non-overkill (bioburden/BI) processes, moist heat sterilizing processes, which are designed and qualified upon the D121-value of a BI, that value is generally in the range of 0.3 to 2.0 min. Bioburden/BI processes require the inherent bioburden resistance to be assessed and to have a lower D121-value. The boil test may be applied to efficiently, expediently, and economically screen for the mere presence of spores. In this application, when employed at sea level, this test can be performed at 100°C for 1 min, while above 2 km in altitude, boiling for 3 min is required (13). A lower temperature could be used; however, it would require greater control and monitoring to ensure temperature uniformity. The thermal challenge under these conditions would not provide any data in terms of D121-value but solely the presence or absence of spores. The uniformity of temperature and duration of incubation at 100°C would not require strict control within this application, because a lower thermal challenge would represent a worst case in which even fewer resistant isolates would be recovered. If isolates are recovered after the 100°C incubation period, these would require further and more rigorous evaluation to establish their D121-value and comparison to the thermal resistance of the BI used to develop and qualify the sterilization cycle. The exact application of the boil test must be carefully considered. If pre-terminally sterilized products are tested without manipulation, any spores screened using this technique exist in their “natural” state, having been generated by a certain species, strain sporulating in the in situ conditions, and have a realistically representative thermal resistance. If a sample is manipulated to recover the resident microflora and spores before using the boil test, those conditions (especially if supporting growth and sporulation) may artificially alter the thermal resistance of the spore and fail to accurately represent the true microbial challenge. This is an especially salient point when considering the evaluation of bioburden recovered from non-product locations or an environmental monitoring isolate.
A common application of the boil test is for the screening of presterilization bioburden thermal resistance, especially in conjunction with the parametric release of terminally sterilized products (21, 22). In this application, pre-sterilization samples are subjected to boiling at 100°C for extended periods of time to more accurately screen and estimate the resistance of the microorganisms that are present. This test is briefly described in USP Moist Heat Sterilization of Aqueous Liquids, <1229.2> (see Table II) (7).
Estimation of Moist Heat Resistance from Boil Test Results*
The origin of the resistance estimates in the table is unknown, but years of successful application of these values have shown their appropriateness. While the method must be acknowledged as somewhat crude, its use for this purpose is nevertheless widespread. The duration of the boiling period can be fixed to a time that conforms to the maximum allowable resistance for the presterilization bioburden, thus becoming a critical element of a bioburden control and monitoring program. The absence of survivors under these conditions provides direct support for the parametric release of product. The same test design, qualification, and control requirements as previously stated for presence/absence screening are necessary for this application of the boil test. Given that this test is designed to target a particular and critical D121-value (or greater), additional controls are necessary to ensure the homogenous thermal treatment of the item. It may be appropriate to ensure that container closure interfaces, seals, and joints (where they exist) are uniformly subjected to the thermal conditions.
Moist Heat D-value Determination
As previously mentioned, moist heat resistance is commonly determined using a BIER that conforms to ISO 18472:2006 (12). This standard outlines the performance requirements for BIER units for several different sterilization methods. When used for steam sterilization, it is restricted to a temperature range of 110°C to 145°C. Those conditions are best suited to heat-resistant mesophilic and thermophilic sporeformers, because those are the only common microorganisms with the ability to survive at those temperatures. In the context of nearly all non-terminal sterilization processes using moist heat, the bioburden and its thermal resistance are ignored. The widespread use of the overkill method and the misuse of the half-cycle method for steam sterilization allow many to simply ignore the bioburden's potential for survival (9).
The use of a BIER vessel outside the ISO 18472:2006 defined operating range is possible; however, a simpler and less expensive means is to determine the resistance of the microorganisms at 100°C. A modest adaptation of the ISO standard operating expectation would allow determination at this temperature, which is more relevant to non–thermophilic spore-former resistance determination. Given the expected increased interest in bioburden resistance, and notwithstanding the careful design requirements, it is possible to perform similar tests at 80°C, and possibly even 60°C, in hot water baths. These temperature ranges are of interest, as vegetative microorganisms have minimal resistance, and treatment at these temperatures may be useful in eliminating these microorganisms as lower temperature sterilization processes become more common. These tests would be performed on a pure strain of a large population in the substrate.
An extensive discussion on apparatus and methods for moist heat-resistance determination of spores is found in Block (23). This content is directed toward the determination of heat resistance in spore populations; however, it could be adapted for use with non–spore-forming microorganisms and at lower temperatures. Execution of D-value studies at 100°C (or below) considering the time–temperature constraints established in ISO 18472:2006 will necessitate some refinement of the boil test. As presently executed, the boil test apparatus used across the industry is both extremely variable and unsophisticated, because it has never been standardized in a compendia or guidance document. Adaptation of existing steam BIER designs providing for precision in both process timing and temperature control at lower temperatures is needed; however, the technical hurdles to doing so are not insurmountable.
Conclusion
Greater patient safety is afforded by terminal sterilization of drugs and devices compared to aseptic processing, because a well-designed, qualified, and controlled process provides the highest level of confidence in the absence of viable microorganisms. Broader adoption of terminal sterilization processes is stymied by the erroneous understanding or automatic adoption of overkill processes, imparting unnecessarily high thermal energy (F0 > 8 min) incompatible with the formulation or a container closure system. Moist heat terminal sterilization processes need to assure the absence of viable microorganisms, fulfilling the desired microbiological quality attribute of sterility, achievable by imparting more modest levels of thermal energy. This is feasible by adopting a bioburden/BI or bioburden approach to sterilization cycle design, qualification, and control principled on meeting the PNSU of ≤10−6. Concomitantly, a bioburden and process control strategy is necessarily commensurate with the design, qualification, and control of non-overkill sterilization cycles. The screening and evaluation of the bioburden is a critical part of this, which necessitates the appropriate design, control, and application of the boil test and BIER vessel to assure the requisite PNSU. Changing the paradigm is expected to increase the use of terminal sterilization and foster adoption of sterilization processes at reduced time–temperature conditions. Under these circumstances, improved knowledge of bioburden resistance becomes essential to process lethality confirmation. The boil test is an important tool in assuring patient safety for terminal treatment with moist heat, especially when less aggressive sterilizing conditions are used. Its potential use varies: (1) screening for the presence/absence of spores; (2) confirming the safety of terminal processes, and (3) determining moist heat resistance for minimally resistant microbial strains. The application of the boil test at temperatures below 100°C could prove beneficial to patient safety as well. Hopefully, this publication has outlined the potential utility of the boil test in varied ways to expand the application of terminal sterilization as well as improve its execution.
Conflict of Interest Declaration
The authors declare that they have no competing financial or nonfinancial interests related to the publication of this manuscript.
Acknowledgement
The authors would like to thank those who anonymously shared their boil test experience with us. It was instrumental in developing the content provided herein, and the authors have endeavored to fulfill our promise and incorporate flexibility throughout our descriptions.
- © PDA, Inc. 2018






