Abstract
The presence of particles in drug products is regulated. These particles may be present before the beginning of the manufacturing process—that is, from the raw materials. To prevent the inclusion of these particles, it is important to understand their composition and origin, so that raw material quality, processing, and shipping can be improved. Thus, it is important to correctly identify particles seen in raw materials. Raw materials need to be of a certain quality with respect to physical and chemical composition and need to have no contaminants in the form of particles, which could contaminate the product or indicate that the raw materials are not pure enough to make a high-quality product. Particles are often seen when handling raw materials because of their different color, size, or shape characteristics. Particles may appear to be very different to the eye than they actually are, so microscope, chemical, and elemental analyses are required for accuracy in proper identification. This paper shows how using three different spectroscopy tools correctly and together can help identify particles from extrinsic, intrinsic, and inherent sources. Particles can originate from material sources such as humans and the environment (extrinsic), from within the process (intrinsic), and as part of the formulation (inherent). Microscope versions of Raman spectroscopy, laser-induced breakdown spectroscopy, and infrared spectroscopy are excellent tools for identifying particles, because they are fast and accurate techniques needing minimal sample preparation that can provide chemical composition, as well as images, that can be used for identification. The micro-analysis capabilities allow for easy analysis of different portions of samples, so multiple components can be identified, and sample preparation can be reduced. Using just one of these techniques may be insufficient to give adequate identification results so that the source of contamination can be adequately identified. The complementarity of the techniques provides the advantage of identifying various chemical and molecular components, as well as elemental and image analyses. Correct interpretation of the results from these techniques is also very important.
LAY ABSTRACT: The presence of particles in drug products is regulated. These particles may be present before the beginning of the manufacturing process—that is, from the raw materials. To prevent the inclusion of these particles, it is important to understand their composition and origin, so that raw material quality, processing, and shipping can be improved. Thus, it is important to correctly identify particles seen in raw materials. Raw materials need to be of a certain quality with respect to physical and chemical composition and need to have no contaminants in the form of particles, which could contaminate the product or indicate that the raw materials are not pure enough to make a high-quality product. Particles are often seen when handling raw materials because of their different color, size, or shape characteristics. Particles may appear to be very different to the eye than they actually are, so microscope, chemical, and elemental analyses are required for accuracy in proper identification. This paper shows how using three different spectroscopy tools correctly and together can help identify particles from extrinsic, intrinsic, and inherent sources. Particles can originate from material sources such as humans and the environment (extrinsic), from within the process (intrinsic), and as part of the formulation (inherent). Spectroscopy uses light to identify materials. This paper shows how using three different spectroscopy tools correctly and together can be used to identify particles from extrinsic, intrinsic, and inherent sources. These were Raman, laser-induced breakdown, and infrared spectroscopies. These techniques are excellent tools for identifying particles, because they are fast and accurate techniques needing minimal sample preparation that can provide chemical composition, as well as images, that can be used for identification. Versions of these that use micro-analyses capabilities allow for easy analysis of different portions of samples, so multiple components can be identified, and sample preparation can be reduced. Each technique has different capabilities that complement each other, and using all three provides the advantage of identifying various chemical and molecular components, as well as elemental and image analyses. However, each of these has limitations and different capabilities that make having them all available for analyses important. Also, it is very important to be able to correctly interpret the results from the instruments.
Introduction
This paper shows how three micro-spectroscopic techniques are used to identify the intrinsic, extrinsic, and inherent sources of possible contaminant particles in raw materials for parenteral drugs and how relying on just one of these techniques would not give adequate results. The presence of particles in drug products is regulated. Particles found in raw materials are at the beginning of manufacturing and should be identified so that they can be eliminated and controlled to assure high-quality final products. Particle contaminants in manufacturing can come from humans and the environment (extrinsic), from within the process (intrinsic), and from formulation components (inherent) (1). Environmental sources can be air and people, and these particles can include insects and materials from employee clothing or personal protection equipment. Intrinsic particles can come from processing equipment and plant environments, and these particles can be rubber, gasket materials, metals, and rust. Paper and cardboard used in packaging have fibers and plastics have fine extrusion aids that can segregate as fine particles. Inherent, extrinsic, or intrinsic water vapor or droplets may also affect the materials, causing clumping or a change in chemistry.
Identification (2) allows evaluation of the severity of the contamination regarding the quality and safety of the product, source determination, and possible elimination. The particles may only look different due to clumping, but the material may be what it is supposed to be. There are regulations regarding particles, including equipment cleaning requirements for processing machinery (3). In addition, injectable drugs must be visually inspected to be essentially particle-free (4), and containers must be visually examined for contamination (5). Raw materials are to be sampled and tested for the absence of contamination to prevent adulteration of finished products (6).
Particles may appear to be very different to the eye than they actually are, so chemical and elemental analyses are required for identification accuracy that will lead to finding the particle source. The tools used should be optimized for accuracy and fast analysis so that the effects on quality can be quickly determined and appropriate corrective steps taken. Microscope versions of Raman spectroscopy, laser-induced breakdown spectroscopy (LIBS), and infrared (IR) spectroscopy are excellent tools for identifying particles in materials because they are fast, require very little sample preparation, and provide complementary information. These techniques can provide chemical composition and images that can be used for identification. They provide the ability for analyses of even very small different portions of samples so that multiple components can be identified with very little sample preparation. The complementarity of the techniques provides the advantage of elemental, molecular, and image analysis. Raman spectroscopy is more sensitive to functional groups with high electron density and crystalline structure than IR spectroscopy. IR spectroscopy is more sensitive than Raman spectroscopy to functional groups with high dipoles, such as CH, NH, and OH. Thus, having both of those molecular spectroscopy techniques available can help find specific components that can indicate the exact source of material. LIBS analysis of the same particles provides additional elemental analyses that can be easily used for clarifying inorganic content, metal analysis, and glass elemental composition. Some examples of using these techniques to analyze particles related to raw materials are presented.
Methods
Raman and LIBS analyses were performed with the Single Particle Explorer life and science (SPE-ls) metal.ID + raman.ID (manufactured by rap.ID Particle Systems GmbH, Berlin, Germany). For Raman, a 785 nm laser with ≤50 mW was used with a spectral range of 300–2000 cm−1 and 5 cm−1 resolution. For LIBS, the pulsed laser was set for 100 μJ and 3 ns. The spectral resolution was 1 nm, and the range was 350–800 nm. Standards libraries and Pearson algorithm were used for spectral identification.
FTIR analyses were performed with a Thermo Scientific Nicolet iN10 Infrared Microscope using a spectral range of 4000–675 cm−1 with 4 cm−1 resolution. Attenuated total reflectance (ATR) spectra were taken with a Ge ATR attachment. Standards libraries and correlation searching were used.
Sample preparation was simply placing the particles on a gold-coated surface for analysis with Raman, LIBS, and IR. In one case, the particle was rinsed with water to remove the raw material coating the surface and allow better spectroscopic analysis.
Discussion
Environmental Sources of Particles (Extrinsic)
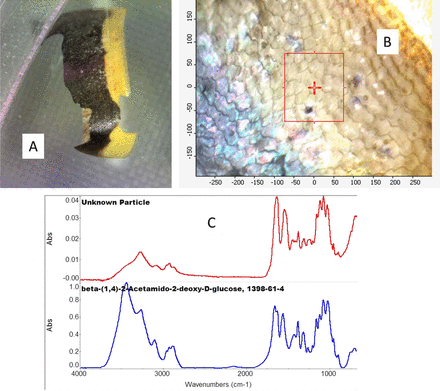
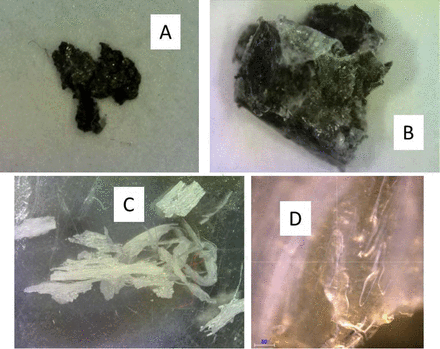
Particles described as “yellow, black, and gray” were found in much of the sodium sulfate raw material. The microscope image indicated that these had scales typical of an insect (see Figure 1). Owing to the color of the particles, the Raman spectra showed mostly fluorescence, so IR was then used for analysis. The IR spectrum of the particle indicated that the material was beta-(1,4)-2-acetamido-2-deoxy-D-glucose from the library search. Library searching can be difficult because the spectra have chemical names or product names, and it is not always clear whether those chemicals are correct or what they might indicate. In this case, beta-(1,4)-2-acetamido-2-deoxy-D-glucose is also known as N-acetyl glucosamine, which is the monomeric unit of chitin. Chitin forms the outer coverings of insects. Thus, the microscope image and the chemical analysis by IR confirm this is part of an insect, and steps must be taken to prevent insects.
A: Photo of the black and yellow particle. B: IR microscope image of the particle showing surface scales. C: IR spectrum of the area in the microscope image (red spectrum) compared to the library spectrum of beta-(1,4)-2-acetamido-2-deoxy-D-glucose (blue spectrum), also known as N-acetyl glucosamine, and chitin. The spectra are offset for clarity.
Part of the Formulation (Inherent)
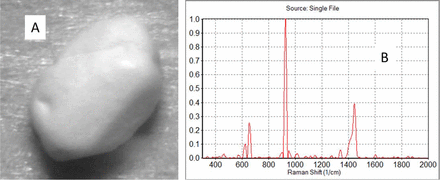
Some particles are visibly detected, because of their different shape or size due to grinding or exposure to moisture. It is important that these be tested, as they could be contaminant materials, and the amount of moisture may be important, even if the particles are the correct material. Figure 2 shows a particle with unexpected size found by visual inspection in the raw material of sodium acetate, but its Raman spectrum indicated it was sodium acetate with no additional components. Thus, the particle was not a contaminant, and the purity of the material was fine. However, it is important to consider the processing of these materials, as the physical characteristic of particle size is important in processing.
A: Photo of the ∼1 mm particle found in sodium acetate. B: The Raman spectrum indicated that it was sodium acetate with no additional components.
Material Processing and Processing Equipment Sources of Particles (Intrinsic)
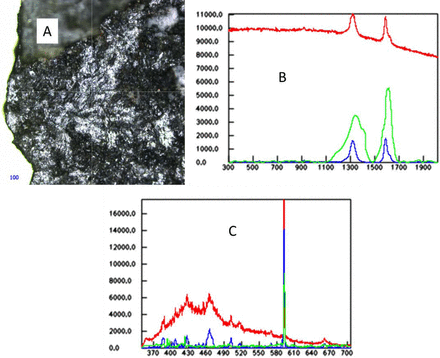
In a sample of sodium phosphate monobasic, a dark particle that appeared from its microscope image to be possibly metal was determined to be charred organic material by Raman spectroscopy. LIBS elemental analysis confirmed the lack of metal and the presence of Na and carbon. LIBS identity of Na and carbon, if done alone, would not give enough information to identify the particle. Sodium phosphate should have no organic material. Thus, the image analysis provided information that was disproved by Raman spectroscopy, and the new interpretation was confirmed by LIBS analysis. The fact that it was charred indicates that it may have come from another use of the processing equipment and that it may not have been sufficiently cleaned. Thus, understanding the chemistry of the materials aids further in finding the source of the material (see Figure 3).
A: Raman microscope image of the particle. B: Raman spectrum of the particle (red spectrum), the baseline corrected spectrum of the particle (blue spectrum), and the library spectrum of charred organic (green spectrum). C: LIB spectrum of the particle (red spectrum), the baseline corrected spectrum of the particle (blue spectrum), and the library spectrum of sodium (green spectrum). Na has a strong band around 590 nm, and the other bands around 425 and 463 nm are due to C.
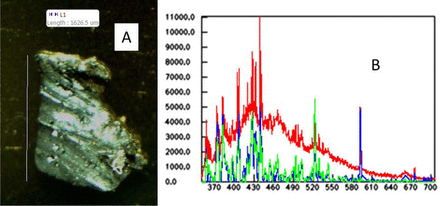
An example of metal found in Na carbonate is shown in Figure 4. In the image, the particle's appearance is shiny and silvery, which suggests metal, and the LIBS analysis quickly identified the metal as steel. Steel would likely come from the processing equipment; thus, the equipment should be inspected to ensure it is in proper condition. In this case, the LIBS confirmed the microscopy result and gave important information about the specific material identity. Raman and IR spectroscopy would not have identified the material because steel does not give Raman or IR spectra.
A: Microscope image of the particle. B: LIB spectrum of the particle (red spectrum), the baseline corrected spectrum of the particle (blue spectrum), and the library spectrum of steel (green spectrum).
Polytetrafluoroethylene (PTFE) is a common contaminant that can come from O-rings. Though the material is often translucent, it can also have pigments. Thus, visual inspection is insufficient for identification. A very dark particle in sodium phosphate was identified as PTFE. Another particle found had both dark and light areas. Other PTFE particles from NaCl appeared white and fibrous like paper by visual inspection but translucent in the microscope image. Based on the visual appearance, the contamination could have been misidentified as paper and only partially identified as polymer based on the microscope image but was correctly and specifically identified as PTFE by the Raman spectroscopy. This indicates that visual inspection is not always sufficient to adequately identify materials based on color and appearance, but chemical analysis must also be done (see Figure 5).
Images of PTFE particles. A and B: Photos of a dark PTFE particle and a dark and light PTFE particle found in sodium phosphate. C: Photo of PTFE particles found in NaCl. D: Microscope image of the PTFE particle found in NaCl.
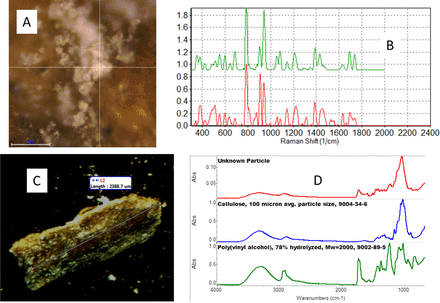
Sometimes particles can appear to be coated with material, and it is important to know if the coatings, as well as the particles, are foreign. Using Raman micro-spectroscopy with its small sampling area, it was possible to determine that a particle from citric acid was coated with citric acid without extra sample preparation. Rinsing the particle with water then allowed IR ATR analysis of the particle. It was identified as cellulose with polyvinyl alcohol, which may be from an adhesive. The complementary capabilities of Raman and IR allowed clear analysis of all components. Raman micro-spectroscopy identified the coating material quickly without special sample preparation, and IR micro-spectroscopy correctly identified the two materials with only minor sample preparation. This shows the advantages of the micro-spectroscopy techniques and the importance of using multiple techniques (see Figure 6).
A: Raman microscope image of the coating on the particle. B: Raman spectrum of the coating (red spectrum) compared to the spectrum of citric acid (green spectrum). (The spectra are offset for clarity.) C: Microscope image of the particle from citric acid. D: IR spectrum of the particle (red spectrum) compared to the library spectra of cellulose (blue spectrum) and poly(vinyl alcohol) (green spectrum).
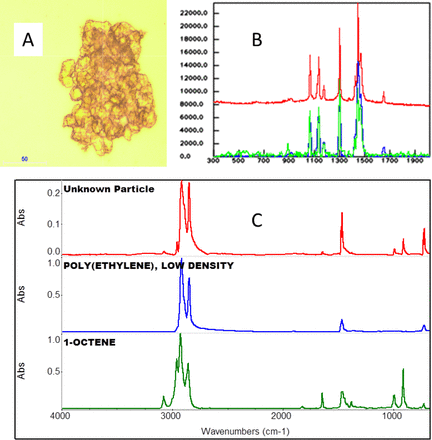
A film/particulate matter found in dimethyl sulfoxide (DMSO) was identified as long-chain hydrocarbon, such as wax or polyethylene. Raman spectroscopy is sensitive to C=C double bonds, and the additional band around 1640 cm−1 is consistent with C=C functionality, not typically in wax or polyethylene. The IR spectrum also indicated polyethylene. IR spectroscopy can be more sensitive to CH vibrational bands than Raman spectroscopy, which can sometimes be used to more specifically ID hydrocarbons. IR library searching of the spectral sections not accounted for by the polyethylene indicated 1-octene, an unsaturated hydrocarbon (C=C), but the bands were weak, and there are many materials with C=C functionality, so the best that can be concluded is the presence of that functional group. Spectral interpretation and the limitations regarding low concentration materials are important in solving the problems of particles. Both wax and polyethylene are not soluble in DMSO, so that is consistent with the identification. From the clumpy appearance of the material it could be an extrusion aid. The use of both Raman and IR gives extra confirmation to the identity of the material (see Figure 7).
A: Raman instrument image of the film/particle. B: Raman spectrum of the particle (red spectrum), the baseline corrected spectrum (blue spectrum), and the library spectrum of polyethylene. The additional band around 1643 cm-1 is consistent with a small amount of C=C functionality. C: IR spectra of the particle (red spectrum) compared to the library spectra of polyethylene (blue spectrum) and 1-octene (green spectrum). (The spectra are offset for clarity).
Conclusions
Visual and microscope analyses were shown to be incomplete methods for identifying possible contaminants in raw materials. Microscope versions of Raman spectroscopy, LIBS, and IR spectroscopy are excellent and complementary tools for identifying particles in incoming raw materials. Sometimes one is better than the others, and sometimes it is important to have them confirm the identity of materials and the lack of other contaminants. These are fast and accurate techniques needing minimal sample preparation that provided chemical composition as well as images that were used for identification. The micro-analysis capabilities allowed for easy analysis of different portions of samples so that multiple components could be identified and sample preparation was reduced. It was shown that relying on only a few of the techniques may not be enough to adequately identify the contaminants. The complementarity of the techniques provided the advantage of identifying various chemical and molecular components as well as elemental and image analyses. These three techniques were shown to be able to identify extrinsic, inherent, and intrinsic particles, and the identifications could lead to improving the processes and quality of the products.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2018