Abstract
Processing equipment involving grinding of two solid surfaces has been demonstrated to induce subvisible particle formation in monoclonal antibody drug product manufacturing processes. This study elucidated potential stress types associated with grinding action to identify the stress mechanism responsible for subvisible particle formation. Several potential stress types can be associated with the grinding action, including interfacial stresses (air–liquid and liquid–solid), hydraulic/mechanical shear stress, cavitation, nucleation of stressed protein molecules, and localized thermal stress. More than one stress type can synergically affect monoclonal antibody product quality, making it challenging to determine the primary mode of stress. Our strategy was to assess and rule out some stress types through platform knowledge, rational judgments, or via small-scale models, for example, rheometer/rotator-stator homogenizer for hydraulic/mechanical shear stress, sonicator for cavitation, etc. These models may not provide direct evidence but can offer rational correlations. Cavitation, as demonstrated by sonication, proved to be quite detrimental to monoclonal antibody molecules in forming not just subvisible particles but also soluble high-molecular-weight species as well as low-molecular-weight species. This outcome was not consistent with that of grinding monoclonal antibodies between the impeller and the drive unit of a bottom-mounted mixer or between the piston and the housing of a rotary piston pump, both of which formed only subvisible particles without obvious high-molecular-weight species and low-molecular-weight species. In addition, a p-nitrophenol model suggested that cavitation in the bottom-mounted mixer is barely detectable. We attributed the grinding-induced, localized thermal effect to be the primary stress to subvisible particle formation based on a high-temperature, spray-drying model. The heat effect of spray drying also caused subvisible particles, in the absence of significant high-molecular-weight species and low-molecular-weight species, in spray-dried monoclonal antibody powders. This investigation provides a mechanistic understanding of the underlying stress mechanism leading to monoclonal antibody subvisible particle formation as the result of drug product processing involving grinding of solid surfaces.
LAY ABSTRACT: Subvisible particles present in therapeutic protein formulations could adversely affect drug product safety and efficacy. We previously illustrated that grinding action of the solid surfaces in some bottom-mounted mixers and piston pump is responsible for subvisible particle formation of monoclonal antibody formulations. In this study, we delved into mechanistic understanding of the stress types associated with solid surface grinding. The approach was to employ several scale-down stress models with known stress types. Protein formulations stressed in these models were analytically characterized for subvisible particles and other degradants. Some commonly known stress types—such as air–liquid interface, mechanical stress, cavitation, nucleation, and thermal effect—were assessed in this study. The stress model yielding a degradation profile matching that of bottom-mounted mixers and piston pump warranted further assessment. Localized, thermal stress proved to be the most feasible mechanism. This study, along with previously published results, may further advance our understanding of these particular drug product manufacturing processes and benefit scientists and engineers in overcoming these development challenges.
- Grinding stress
- Monoclonal antibody
- Subvisible particles
- HMWs
- LMWs
- Cavitation
- Sonication
- Thermal stress
- Spray drying
1. Introduction
We previously demonstrated that some monoclonal antibody (mAb) drug product manufacturing unit operations, including mixing and aseptic filling, are likely to cause the formation of subvisible particles (SvPs) in mAb drug substance formulations (1). Later we attributed the grinding stress associated with unique equipment designs of these unit operations to be the primary root cause of SvP formation. The action of such equipment always involves one moving solid surface touching (i.e., grinding) a stationary solid surface (2). Many single-use, bottom-mounted mixers (3⇓–5), for example, contain such grinding surfaces between the coupling of the impeller and the drive unit. The piston pump presents another source of grinding interaction between the piston and the housing unit that results in the formation of SvPs during the filling operation of mAb products (6⇓–8). Formation of mAb SvPs due to the grinding action was demonstrated not only within a tight gap (e.g., bottom-mounted mixers and piston pump) but also in the open environment, where mixing is achieved via spinning a magnetic stir bar against the solid surface of the mixing container (1, 2, 9, 10).
However, underlying stress mechanisms/types contributing to mAb degradation are not yet fully understood except for one general observation. For all the mAb molecules/formulations tested in these systems, only SvPs (i.e., insoluble aggregates) were observed, while no soluble aggregation was detected. This finding suggested that whatever the stress type is, it might only apply to localized, micro-volume (11). Assessing general stress types associated with protein liquid agitation/mixing may benefit linking these stress mechanisms to the grinding action. Kiese et al. (9) observed visible and subvisible mAb particles as the result of stirring using a magnetic stir bar, and they named several stress types as the likely culprits. These stress types included (a) shear, (b) interfacial effects (liquid–solid interfaces), (c) cavitation, (d) local thermal effects of stirring due to the friction between the stir bar and the bottom of the container surface, and (e) rapid transportation into solution of aggregated or adsorbed protein species, which can serve as nucleating sites for further aggregation within the bulk. There is another nucleation theory proposed by Tyagi et al. with a piston pump study (8), in which the grinding of the piston and the housing shed stainless steel nanoparticles that served as nucleating sites for mAb aggregation to form SvPs.
Shear stress has been widely speculated to play a role in causing protein aggregation, although many studies concluded that shear stress alone is not the main factor for protein aggregation, at least for small, globular proteins (12⇓–14). The effect of shear stress became prominent only when air–liquid interfaces (15⇓–17) or liquid–solid interfaces (18, 19) were present. Whether a high-shear environment may affect larger proteins, such as mAbs, has not yet been extensively investigated. A study by Bee et al., which applied high shear to a concentrated mAb using a capillary rheometer and a parallel-plate rheometer, suggested that shear alone did not cause aggregation (20).
Cavitation is a unique fluid dynamic phenomenon of forming vapor cavities in a liquid due to pressure change. A sudden pressure drop causes the formation of cavities (bubbles) that quickly implode when exposed to high pressure. This continuous cavity forming and collapsing generates intense shock waves. These shock waves cause damage to the solid surfaces and the liquid because the imploding cavities create localized, short-lived hot spots with high temperature and pressure (11, 21, 22). Furthermore, the collapsing bubbles may generate hydrogen/hydroxyl free radicals (23, 24), which can react with proteins, resulting in chemical, structural, and functional changes (11, 25). Ultrasound is known to create cavitation bubbles.
It is intuitive to assume that grinding of two solid surfaces causes friction that may generate heat. However, the intensity of the heat and its duration are unknown and difficult to predict (26). Furthermore, these hot spots are confined to an extremely small volume or area, making it a daunting task to find a stress model to investigate the grinding-induced thermal effect.
The theory of nucleation at liquid–solid interfaces was extensively studied by Bee et al. (27), who demonstrated the different effects on protein adsorption and aggregation when a mAb is exposed to microparticles of glass, cellulose, or stainless steel. In a separate study, Tyagi et al. concluded that stainless steel nanoparticles shed by a rotary piston pump can serve as heterogeneous nuclei for the formation of protein microparticles (8). However, this hypothesis could not be confirmed by others (1, 7).
The study presented here investigated all stress types discussed above using experimental models with identified stress types in order to link the stress mechanisms associated with grinding between two solid surfaces. In this study, mixed mAb solutions were characterized using visual inspection, turbidity by UV spectrophotometer, SvP count and morphology analysis, and soluble aggregates by size-exclusion high-performance liquid chromatography (SEC-HPLC). Three techniques of particle analysis were used: light obscuration (HIAC Royco), electrical conductance (Beckman Coulter MultisizerTM coulter counter®), and micro-flow imaging (MFI). Fourier transform infrared (FTIR) spectroscopy was also used to assess any impact of grinding stress on mAb high-order structure.
2. Materials and Methods
Four mAb drug substance formulations, which included one of the three mAb molecules formulated in two different stabilizers (mAb A1, mAb A2, mAb B, and mAb C; Genentech Inc., South San Francisco, CA, USA), were used to determine the impact of various stress models on product quality (Table I).
Monoclonal Antibody Drug Substance Formulations Used in Stress Mechanism Identification Studies1
2.1. Experiments Using Different Processing Models
2.1.1. Mixing Experiments:
The mixing experiments utilized a customized Mavag mixer (Mavag AG, Neunkirch, Switzerland), which was supplied with a 10 L glass container (2.5 L of minimum operating volume) and equipped with a MDB 50 impeller as described previously (1). As described previously (2) the impeller of this mixer could be replaced by a bladeless impeller, and a cylindrical divider (a piece of plastic tube) could be added to the top of the impeller to confine some of the mAb formulation liquid to the top region of the impeller.
2.1.2. Shear Stress Experiments:
Two shear stress models were used to assess different types of shear stress on mAb formulations:
Double-gap rheometer: An Anton Paar rheometer fitted with a double-gap cylinder stainless steel measuring system (DG26.7) was used, and analysis was performed using Rheoplus software (Aanton Paar GmbH, Graz, Austria). The gaps between the rotating and the stationary cylinders were 0.419 mm and 0.469 mm (inner gap and outer gap, respectively) based on the dimension of R1 = 11.910 mm, R2 = 12.329 mm, R3 = 13.329 mm, and R4 = 13.788 mm (see diagram in Table II). In the sample container, a liquid sample (volume of 3.8 mL) was sheared for 60 min at 660 rpm (shear = 2000s−1).
Rotor-stator homogenizer: A Virtishear homogenizer (Virtis, Tempest IQ) consisting of a digital display microprocessor control, an overhead drive unit, and a homogenizing shaft (160 mm) was used. The shaft tip was a rotor/stator assembly capable of generating fine dispersions (see diagram in Table II). This assembly resembled a concentric cylinder shear device, but it was actually an open system. The dimensions of the rotor and the stator were as follows: the rotor outer diameter, ROD = 7.4 mm and the rotor height, RH = 18.0 mm; the stator inner diameter, SID = 8.2 mm and the stator height to the base of the rotor, SH = 22 mm. The experiment was performed on 20 mL mAb solution in an 18 × 150 mm borosilicate glass cell culture tube for up to 60 min at 5000 rpm. The homogenizer rotor-stator probe was submerged 1.5 inches deep into the solution.
Stress Models and Anticipated Stress Mechanisms Occurring in the Gap within Impeller/Drive Unit Assembly and Piston/Housing Coupling
2.1.3. Cavitation Experiments:
Cavitation was created using a sonicator (Q55, Qsonica, Newton, CT, USA) with a 1/8″ diameter microtip titanium alloy probe. A 2 mL liquid sample in a custom container was sonicated for up to 60 min under the following conditions: power at 55 watts, frequency at 20 kHz, and amplitude of 50% (∼90 μm up and down in linear movement). The custom container is capable of cooling the sample using a water bath, and the mAb solution temperature was controlled at 12 °C.
2.1.4. p-Nitrophenol (PNP) Experiments To Investigate Presence of Cavitation:
PNP compound (MP Biomedicals, Solon, OH, USA) was dissolved in the same formulation as mAb A2 to make a 0.02 mg/mL working solution. The solution was stressed using several stress models under similar conditions as mAb formulations (i.e., mixing by Mavag mixer at 200 rpm for 60 min, shear by rotor-stator homogenizer as described in section 2.1.1 (b), and sonication as described in section 2.1.3). Changes in the absorbance spectra were analyzed using an Agilent HP 8453 UV-visible spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Local Amax at 318 nm wavelength was used to compare the impact of the various stresses on PNP solution.
2.1.5. Spray-drying Experiments:
Spray drying was used as a micro-droplet drying model. To separate the dehydration effect, freeze drying of the same formulation was also performed as a control.
A laboratory-scale spray dryer (Anhydro MS-150, SPX Flow Technology Systems, Inc., Elkridge, MD, USA) was constructed mostly of stainless steel with heated insulation for the drying chamber and cyclone. Approximately 200 mL of the mAb solution was spray-dried using drying air of ∼190 °C (inlet temperature) at a rate of 38 kg/h. The liquid was fed at a rate 15 mL/min to result in ∼90 °C measured as the outlet temperature of the drying air. The liquid was atomized through a two-fluid nozzle using atomizing gas at a flow rate of 4 kg/h. To evaluate the effect of atomization alone, the protein solution was atomized from the two-fluid nozzle (air flow rate of 4 kg/h) directly into a glass beaker.
The mAb solutions were also freeze-dried to compare with the spray-dried counterparts. The mAb solutions were distributed (1 mL) into 2 cm3 glass vials and partially sealed with lyophilization butyl rubber stoppers. The vials were loaded on pre-chilled shelves at –50 °C in a lyophilizer (model Lyomax 2, BOC Edwards, Tewksbury, MA, USA). During the primary drying cycle, the frozen mAb solutions were dried for 40 h at 100 mTorr and at a shelf temperature of 25 °C. Secondary drying was performed for 12 h at 35 °C at 100 mTorr. The total lyophilization cycle time was approximately 60 h.
2.2. Product Quality and Process Performance Characterizations
2.2.1. Particle Count and Distribution Analyses:
Light obscuration measurements for SvP counts and distribution were performed using a HIAC Royco Liquid Particle Counting System (Hach Company, Loveland, CO, USA) following the same procedures reported previously (1).
The electrical sensing zone principle for SvP counts and distribution by Coulter Counter® Multisizer 4 (Beckman Coulter, Inc., Fullerton, MA, USA) was used following the same procedures reported previously (1).
Dynamic imaging particle analysis was performed using the MFI 5200 Particle Analyzer (Protein Simple, Santa Clara, CA, USA) for SvP count, distribution, and to decipher particle morphology differences. The instrument was equipped with a 100 μm silane-coated flow cell, 5× objective, monochrome camera, and a peristaltic pump. The instrument was autofocused using 10 μm Duke size standards and controlled with the MFI View software version 2R3.0.1.24.2461, while MVAS version 1.3 was used for image processing.
2.2.2. Visual Inspection:
The samples were visually inspected in a light box against white/black background for the presence of visible particulates and changes in color and opalescence.
2.2.3. Turbidity:
Opalescence of the stressed solutions without dilution was measured in a 1 cm path-length cuvette using a UV spectrophotometer (model 8453, Agilent Technologies). The test samples were blanked against purified water. The absorbance was recorded at 340, 345, 350, 355, and 360 nm wavelength, and the average of the absorbance readings was reported as the turbidity of the samples.
2.2.4. Size-Exclusion Chromatography (SE-HPLC):
The analysis utilized a G3000SWXL column, 7.8 mm ID × 30 cm, 5 μm (TOSOH BioScience, King of Prussia, PA, USA), run on a high-performance liquid chromatography (HPLC) system (model 1200, Agilent Technologies). The mobile phase was 0.2 M potassium phosphate and 0.25 M potassium chloride at pH 7.0 for mAb A. Chromatography was run isocratically at a flow rate of 0.5 mL/min for 30 min. The column temperature was maintained at ambient temperature, and the eluent absorbance was monitored at 280 nm. The mAb A sample (20 μL) was injected directly without dilution. The same method was used for analysis of mAb B and mAb C except that the samples were diluted to 10 mg/mL using formulation buffer, and 20 μL of sample (∼200 μg) was injected. In addition, 0.1 M potassium phosphate at pH 6.8 was used as the mobile phase for mAb B, and 0.2 M potassium phosphate and 0.25 M potassium chloride at pH 6.8 was used as the mobile phase for mAb C.
2.2.5. FTIR Spectroscopy:
Testing was performed using a Vertex 70 FTIR spectrometer equipped with a BioATRcell II Type A729/Q accessory (Bruker Optics, Inc., Billerica, MA, USA; for liquid samples) or a VideoMVPTM Single Reflection ATR Microsampler (Harrick Scientific Products, Inc., Pleasantville, NY, USA; for solid pellet samples). Solid pellet samples were separated from the supernatant solution by centrifugation at 10,000 rpm for 60 min at uncontrolled room temperature using a Tomy MicroTwin centrifuge (Tomy Kogyo Co., Tokyo, Japan). The autosampler temperature (for liquid samples testing only) was maintained at 25 °C by a Huber Ministat 125 thermoregulator (Peter Huber Kältemaschinenbau AG, Offenburg, Germany). Before each undiluted sample was measured, an appropriate blank was scanned and subtracted from the sample spectrum. The measurement (120 scans) was done with an optical resolution of 4 cm−1. The spectra were normalized against the amide I band to compare any gross changes in secondary structure.
3. Results and Discussion
Determining the type of the stress associated with grinding action between two solid surfaces is a daunting task because the stress only applies to localized, microscopic areas/volumes, and several stress types may synergically act on the protein simultaneously. Thus, the approach in this study was to evaluate models with known stress types and link their relevance to the grinding zone within the impeller and drive unit assembly or piston/housing assembly per analytical testing outcomes. If a model contains more than one stress type, these stress types need to be further dissected for analysis. Analytical tools include visual inspection, UV spectrophotometer (turbidity), SvP analyzers (particle count, distribution, and morphology), SEC-HPLC for soluble aggregates (HMWS) and fragments (LMWS), and FTIR for conformational changes.
3.1. Stress Types
Several types of stress are likely associated with grinding action (Table II). These stress types may involve (i) air–liquid interfacial stress, (ii) hydraulic shear stress, (iii) mechanical impact, (iv) liquid–solid interface–induced nucleation, (v) cavitation, and (vi) friction-induced localized, thermal effect. Of these stress types, (i) to (iii) are unlikely to occur within the grinding zone (i.e., within the gap of impeller/drive unit assembly and the piston/housing coupling).
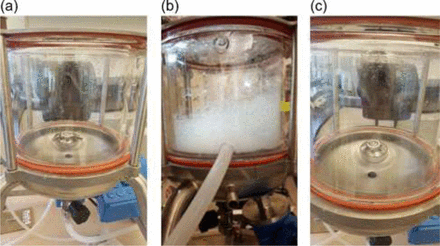
Air–liquid interfacial stress is relatively easy to assess. After 2.5 L of mAb A1 was mixed for 60 min at 200 rpm in the Mavag mixer, substantial foaming was generated (Figure 1b as compared with Figure 1a, which shows the solution before mixing). In contrast, the same experiment performed using a bladeless impeller (same design of impeller/drive unit coupling) showed no obvious foaming (Figure 1c). Despite the contrast in foaming, the SvP count of the two solutions were comparable, 4.67 × 105 ± 8.71 × 104 vs. 5.03 × 105 ± 2.77 × 104 particles/mL (by Coulter Counter®). Dissipation of the foam following mixing with the bladed impeller did not increase SvP counts in the solution, indicating that the foam does not act as a trap for the SvP formed. As the generation of SvPs was previously attributed to grinding between the surfaces of the impeller and drive unit assembly (2), it suggested that, for mAb formulations tested, air–liquid interfacial stress (i.e., foaming) plays no obvious role in SvP formation, and mAbs may have been effectively protected by a surfactant at concentrations above their critical micelle concentration level in the formulation. Given that the presence of air bubbles within the gap of the Mavag impeller/drive unit assembly or the piston/housing coupling is unlikely, it is reasonable to rule out air–liquid interfaces from the stress contribution in this study.
Hydraulic shear stress is a common internal stress acting on the liquid within the boundary layer adjacent to a solid surface. This shear stress is prominent within the gap between the impeller/drive unit assembly and the piston/housing coupling as the impeller and the piston rotate. This shear was simulated previously using rheometers (i.e., double-gap, cone-plate, and plate-plate) (2). Despite worst-case surface area to volume ratio and shear stress, the mAb formulation that was stressed in these rheometers did not form significantly more SvPs compared with the unstressed control, suggesting that hydraulic shear stress alone did not affect mAb SvP formation. This conclusion was consistent with the findings of others (15⇓–17, 20). Thus, hydraulic shear stress alone can also be excluded from consideration in this study.
Mechanical impact is a less reported stress type, at least for protein drug product processing. As high-velocity liquid collides with solid surfaces, the liquid suffers velocity reduction and may convert its kinetic energy into internal energy in the form of deformation or heat. Mechanical impact occurs in a highly turbulent environment, often associated with circulation movement. Although impeller mixing can create turbulence and circulation, the presence of turbulence and circulation within the gap of impeller/drive unit assembly and the piston/housing coupling is unlikely.
Although stress types (i) to (iii) are unlikely to occur in the systems of our interest, it is still possible that they may synergize with other types of stress. Stress types (iv) to (vi) are considered more likely to occur within the grinding zone (or their effects are less certain to be excluded from consideration).
Liquid–solid interface–induced nucleation may occur in two forms. Protein molecules may be absorbed to and undergo conformational changes at solid surfaces. Liquid flow and shear stress at solid surfaces later sweep these absorbed molecules back into solution bulk. These disturbed protein molecules may serve as nucleation sites for interaction with other similarly disturbed molecules (9). Another form of nucleation is specifically associated with grinding. Grinding surfaces may shed solid particles, most likely in the form of nanoparticles. These nanoparticles may serve as heterogeneous nucleation sites for protein aggregation (8). These stress mechanisms are rational and require experimental confirmation.
Cavitation is generally an undesirable fluid dynamic phenomenon that occurs during drug product processing. A common form of cavitation occurs due to rapid velocity change of a moving solid, resulting in a sudden pressure drop at the solid surface. Vapor cavities (or bubbles) form when the pressure drops below the vapor pressure of the liquid. These cavities may implode after subsequent exposure to higher pressure. Repeated cavity implosion results in shock waves and hot spots. These short-lived hot spots are localized regions of extremely high temperature and pressure (up to thousands of degrees Kelvin and hundreds of atmospheres of pressure) (11, 21, 22). However, it has not yet been established whether grinding action causes cavitation.
Like the hot spots caused by cavitation, the friction due to grinding of two solid surfaces may lead to localized, high-temperature hot spots as well. However, these hot spots are transient and confined to a very small region. It is unlikely to observe protein quality changes if a liquid bulk (e.g., a few milliliters in a vial) is exposed to a high temperature for a short period of time, while a long exposure may yield protein quality changes irrelevant to the grinding event. Thus, the impact of hot spots on mAb degradation can only be verified using a model capable of exposing high temperature to a very small volume for a short period of time.
Visual inspection of mAb drug substance solution: (a) before mixing, (b) after mixing with the bladed impeller, and (c) after mixing with the bladeless impeller.
3.2. Stress Models
Several stress models, along with likely involved stress mechanisms, used in this study are also listed in Table II.
Double-gap rheometer: This model features a large surface area (the combined surface area of the rotational cylinder and stationary cylinders) and a small sample volume (3.8 mL). Its surface area to volume ratio is more than 3000 times higher than that of the gap within the impeller/drive unit assembly of the Mavag mixer, 3.632 m2/L vs. 0.001 m2/L (see Table IX in Reference 2). Under comparable shear conditions (shear rate and tip speed of the rotating cylinder), this large surface area to volume ratio provides a worst-case model for investigating the absorption-based nucleation mechanism associated with the liquid–solid interfacial stress.
Rotor-stator homogenizer: The rotor and the stator are concentrically assembled. When the rotor (inner cylinder in the form of two blades) rotates, the assembly draws the liquid in from the bottom and sends the liquid out through the four circular holes equally spaced on the stator (outer, stationary tube) for circulation. This device combines several shearing mechanisms for mixing as well as size reduction of solids and liquid (emulsification)—hydraulic shear stress, mechanical impact, and cavitation (28). However, the presence of cavitation by this homogenization method is not the major mechanism. Table II also lists air–liquid interface as one of stress types present in this processing device, although liquid turbulence was minimized. No obvious turbulence was observed in this study because the volume of the liquid sample exceeded the recirculation capability of the homogenizer.
Sonicator: Ultrasound sonication is well known for generating cavitation. Quick vibration of the sonication probe creates cavities/bubbles. No obvious turbulence was observed during sonication in this study. Air–liquid interface associated with cavitation bubbles is listed in Table II as one of the stress types.
Incubation for nucleation: Liquid samples stressed in the double-gap rheometer, rotary piston pump (10× recirculation), and the Mavag mixer were incubated at 25 °C for up to 48 h to corroborate the two nucleation hypotheses due to liquid–solid interfacial interactions (Table II). The double-gap rheometer provided very high stainless steel surface area (relative to sample volume) for protein adsorption/desorption where the proteins might undergo conformational changes or aggregate. The adsorbed protein molecules or aggregates could be swept back into solution by hydraulic shear force to serve as nucleation sites for further aggregation. In the Mavag mixer, the impeller grinded against the drive unit to shed ceramic particles as demonstrated previously in mixing a placebo solution (1). Ceramic particles may serve as heterogeneous nucleation sites for protein aggregation (8). In the piston pump, stainless steel nanoparticles could be shed during movement of the piston within the pump housing (6⇓–8). Incubation at a temperature above 2–8 °C should accelerate nucleation and protein aggregation.
Spray drying: As suggested earlier, grinding-induced friction may generate heat. This heat is transient and confined to micro-volumes/areas. Both factors are important for model selection. Even when heating a reasonably small liquid volume (e.g., 1 mL), a duration that is too short may yield no detectable degradation, whereas a duration that is too long may result in irrelevant degradation routes. Spray drying is a rapid dehydration process featuring atomizing liquid into micron-sized droplets (e.g., ∼10 μm) that are subsequently dried in hot air. The temperature of the hot air entering the drying chamber can be controlled at nearly 200 °C. The duration of the drying time (from liquid atomization to dry particle collection) is only a few seconds, depending on the scale of the spray dryer (29⇓⇓⇓⇓–34). The droplets may, however, experience such a high temperature only at the beginning of the drying process. Spray drying appears to meet all the criteria as a suitable model for investigating the localized thermal stress. However, there are other stress types also acting on the protein: shear stress/air–liquid interface (during atomization) and dehydration stress. Liquid atomization without drying can be performed to evaluate stress from shear and air–liquid interface. Lyophilization of the same formulation can be used as a control to assess the impact of dehydration stress.
3.3. Impact of Stress Models and mAb Characterization
mAb A1 and mAb A2 formulations were stressed using a bladeless Mavag mixer (with inner reservoir), a rotor-stator homogenizer, and a sonicator. In all cases, stress conditions yielded no visible agitation-induced turbulence. Stressed samples were characterized for turbidity, particle analysis by MFI, and size variants by SEC-HPLC. Table III summarizes all test results.
Characterization of mAb Formulations (mAb A1 and mAb A2) Stressed in Different Stress Models
3.3.1. Turbidity:
All stressed protein samples became more turbid after processing, particularly after mixing in the bladeless Mavag mixer (solution in the inner reservoir). The sucrose formulation (mAb A1) was observed to become less turbid when the samples were stressed using a rotor-stator compared with the NaCl formulation, while the opposite was the case for formulations stressed using sonication. This might be due to differences in the primary mode of stress in each model.
3.3.2. Particle Analysis:
MFI particle analysis showed a similar trend: In all stress models, there was a substantial increase in SvP counts relative to the unstressed control. There was no obvious difference in the average particle size among these stress models. The impact of the stress models on particle counts of mAb A1 and mAb A2 (formulations containing sucrose vs. NaCl, respectively) was also not apparent. Given that sucrose is known as a better protein stabilizer than NaCl, it might suggest that the stress rendered by these stress models are too formidable to be overcome by formulation optimization. However, formulation appeared to affect particle morphology.
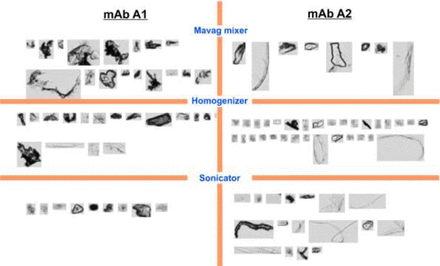
Figure 2 shows the morphology of particles (only for particles of ≥20 μm by MFI) generated by the three stress models for both formulations. In general, more fibrous particles were observed in the formulation containing NaCl, while more dense particles were present in the formulation containing sucrose. Joubert et al. (10) also performed MFI particle morphology characterization on particles of a mAb produced by various stress techniques. The researchers did not link particle morphology to stress types, but they did link particle size to the extent of stress—the more severe the mechanical stress, the larger the particle size. Randolph et al. (11) indicated that cavitation-induced particle size and morphology resemble the particles produced during the mechanical stresses (agitation, stirring, etc.) described by Joubert et al.
Morphology of SvP particles (only SvP ≥20 μm are shown) formed by stressing mAb A1 and mAb A2 formulations using the Mavag mixer, rotor-stator homogenizer, or sonicator, as assessed by MFI.
Overall, there is no clear correlation between stress types and particle morphology for the three modes of stress (mixing, homogenizing, and sonicating) and two mAb formulations (containing sucrose or NaCl) investigated in our study.
3.3.3. Size Variants (SEC-HPLC):
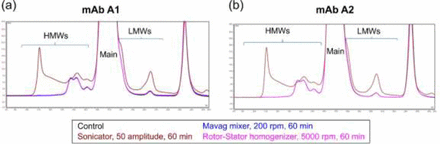
Table II lists cavitation and mechanical impact stress as the most probable stress types contributing to SvP formation in rotor-stator homogenization, while cavitation has an apparent effect in the sonication model. As mechanical impact is unlikely to occur in the gap within the Mavag mixer's impeller/drive assembly and cavitation is the stress mechanism shared in both stress models, stressed formulations were further characterized for size variants (by SE-HPLC). Figures 3a and 3b display the overlay of SE-HPLC chromatograms of the mAb A1 and mAb A2 drug substance formulations, respectively. These two liquid formulations were stressed in the Mavag mixer, the rotor-stator homogenizer, or the sonicator. As shown in Table III, the size variant attributes (monomer, HMWs, and LMWs) of mAb A1 and mAb A2 after being stressed in the Mavag mixer and the rotor-stator homogenizer were identical to those of the unstressed control. The size variant attributes of the sonicated mAb A were, however, very different, with significant increases in both HMWs (∼5%) and LMWs (∼3%) compared with the control sample. Given that particle analyzers (MFI and Coulter Counter®) are more sensitive than SE-HPLC analysis in detecting the amount of aggregates relative to the overall protein content in a given solution, the relative protein amount in the form of particulates is significantly lower than that in the form of soluble aggregates; calculation confirmed the amount of protein in the form of SvPs is approximately 0.01% of the entire protein mass (35⇓–37). Thus, the more-than 7% monomer decrease associated with sonication-induced stress suggested that cavitation adversely affects protein not only microscopically (stress acting on a very small volume/area) but also macroscopically (stress acting on a greater volume). This difference may suggest that cavitation is unlikely to be the primary stress type in the gap within the Mavag mixer's impeller/drive unit assembly. Whether cavitation is present or absent in these stress models requires more evidence.
An overlay of SEC chromatograms comparing (a) mAb A1 and (b) mAb A2 formulations stressed by the Mavag mixer, rotor-stator homogenizer, or sonicator.
3.3.4. Cavitation and PNP Degradation:
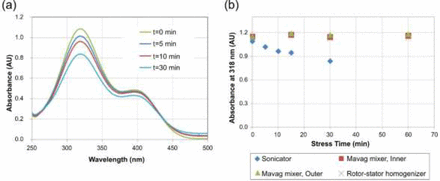
PNP is an azo dye, known to degrade upon exposure to acoustic cavitation resulting from ultrasonic irradiation (38). A large amount of energy released during cavitation produces intense local heating and builds high pressure, which facilitate the formation of free radicals, such as hydroxyl radical (ÖH). These hydroxyl radicals can chemically react with PNP. A change in the concentration of PNP can be readily monitored using UV spectrophotometry. Thus, PNP is a convenient tool to demonstrate the presence or absence of the cavitation phenomenon (39, 40). PNP in the formulation buffer of mAb A1 was used to assess whether cavitation was present during solution mixing using the stress models listed in Table II (Mavag mixer, rotor-stator homogenizer, ultrasound sonicator). Analysis of the UV-visible spectra showed that absorbance (at primarily 318 nm wavelength) decreased with increasing sonication time (Figure 4a). In contrast, PNP concentration remained constant in samples stressed in the Mavag mixer (both inner and outer reservoirs) as well as the rotor-stator homogenizer (Figure 4b). This result suggested that cavitation is unlikely to be the primary mode of stress in the gap within the Mavag mixer's impeller/drive unit assembly or piston/housing assembly in rotary piston pump.
Using p-nitrophenol (PNP) to assess cavitation from mixing, homogenizing, and sonicating: (a) UV-visible spectrum of 0.02 mg/mL PNP stressed by sonication; (b) absorbance at 318 nm for PNP solution stressed by sonication, in the inner reservoir of the Mavag mixer, in the outer reservoir of the Mavag mixer, and by the rotor-stator homogenizer.
3.3.5. Nucleation Hypotheses:
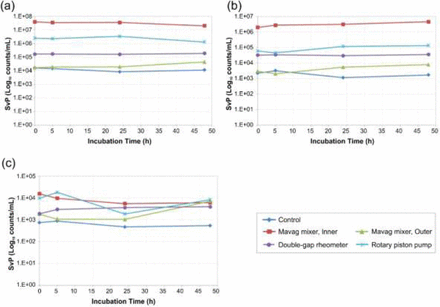
There are two nucleation hypotheses. Grinding of solid surfaces can shed nanoparticles of the solid material. These nanoparticles can serve as nucleation sites for protein aggregation. Due to the difference in the construction of the material, grinding of the impeller/drive unit ceramic bearing coupling in the Mavag mixer can shed ceramic nanoparticles (1), while grinding of the piston/pump housing in a rotary piston pump can shed stainless steel nanoparticles (7, 8). The second hypothesis involves the interaction of protein molecules that are adsorbed to solid surfaces, conformationally deformed, and desorbed from solid surfaces by flow turbulence. To test these two hypotheses, mAb A1 formulation was stressed by mixing in the Mavag mixer (bladeless impeller at 200 rpm), 10 passes through the piston pump (i.e., 10× recirculation), and controlled shear in a double-gap rheometer. The stressed solutions were then filled into 1 cm3 vials (∼1 mL/vial) and incubated at 25 °C for up to 48 h. SvP count and distribution of all stressed samples were determined using a Multisizer 4 Coulter Counter® particle analyzer. The incubation results are presented according to SvP size range: 1.5–3.0 μm (Figure 5a), 3.0–6.0 μm (Figure 5b), and 6.0–30.0 μm (Figure 5c). There was no obvious increasing trend in SvP count after incubating the stressed material for up to 48 h, suggesting that the nucleation hypothesis is unlikely to be the primary stress mechanism for grinding between two solid surfaces.
Comparison of changes in SvPs at various size ranges over incubation time at 25 °C as determined by Multisizer 4 Coulter Counter® particle analyzer: (a) 1.5–3.0 μm, (b) 3.0–6.0 μm, (c) 6.0–30.0 μm. The mAb A1 formulation was subjected to various modes of stress: control; mixed in the inner reservoir of the Mavag mixer and outer reservoir of the Mavag mixer; sheared in a double cylinder rheometer; recirculated through a rotary piston pump.
3.3.6. Localized, Thermal Stress:
Spray drying can subject liquid droplets with micrometer-sized diameters to high temperature for a short period of time, which satisfies the criteria as a localized, thermal stress model. The manufacturing-scale spray dryer used in this study was recently reported to be feasible for mAb powder preparation (33, 34). After powder reconstitution, the mAb was characterized for protein size variants (by SE-HPLC) and SvP analysis. mAb A1 and mAb A2 formulations were found to be infeasible for spray drying due to formulation stickiness (sucrose) and excipient crystallization (NaCl). Thus, the mAb B and mAb C formulations, which were successfully spray-dried previously (34), were used as the model drug substance formulations in this study.
Both formulations were stressed by mixing in the modified Mavag mixer (inner and outer reservoirs) or by spray drying, and test results are summarized in Table IV. Both spray drying and mixing in the inner reservoir of the Mavag mixer generated a significant amount of SvPs relative to the unstressed control. Spray drying involves multiple stress types: air–liquid interfacial stress and shear stress associated with atomization, dehydration stress, and heat/thermal stress. To differentiate, both mAb solutions were atomized (without drying) and lyophilized (low-temperature dehydration), respectively. It is apparent from Table IV that stress from atomization has a minimum effect on SvP formation while dehydration stress (by lyophilization) has a minor impact on forming SvPs. Thus, high levels of SvPs observed in spray-dried powders could be attributed to stress associated with heat exposure (drying air inlet temperature at close to 200 °C).
Characterization of mAb Formulations (mAb B and mAb C) Stressed by Spray Drying or Mixing
All samples (stressed and controls) were also tested for HMWs and LMWs by SEC-HPLC (Table IV). There was only a small increase in HMWs (<1%) for dehydrated samples without obvious differences between spray-dried and lyophilized product, while there was no change in LMWs. This suggested that soluble aggregation is mainly caused by dehydration but not necessarily heat stress. With samples stressed in the Mavag mixer also showing only SvPs (no change in HMWs and LMWs), the hypothesized localized, thermal effect arising from grinding of solid surfaces in the Mavag mixer could be linked to the heat stress in the spray-drying model.
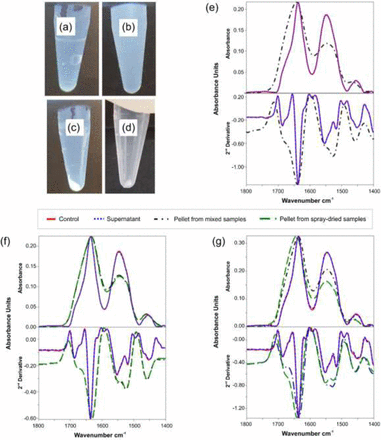
Temperature exposure may cause structural changes of the mAb, and the extent of change may depend on the type and intensity of the stress applied. FTIR was used to probe gross changes in the secondary structure of mAbs in both mixed and spray-dried samples. The control samples (i.e., before mixing or spray drying) of the three mAbs used in this study were clear to slightly opalescent (Figure 6a). After mixing in the Mavag mixer or spray drying, solutions turned more turbid (Figure 6b). Particles were separated from the supernatant solution by centrifuging the samples at 10,000 rpm for 60 min (Figure 6c and 6d), and the resulting pellet and liquid were analyzed using FTIR. The FTIR spectra of the liquid and the pellet were compared by normalizing the spectra against Amide I band (Figure 6e through Figure 6g). A second derivative of each spectrum was also determined. The overlays indicate no gross changes in higher order structures between the control and the supernatant solution, while a substantial change between the precipitated pellet material and its respective supernatant solution is observed. This is indicated, for instance, by a decrease in β-sheets, as shown in Table V. The secondary structure perturbation by mixing and spray drying is not overly different and might suggest that both processes may share a similar stress mode (i.e., localized, thermal stress).
Visual inspection and FTIR analysis of mAb A2, mAb B, and mAb C formulations: photographs of (a) mAb A2 control, (b) mAb A2 after mixing with Mavag mixer at 200 rpm for 60 min (inner reservoir), (c) sample b after centrifugation at 10,000 rpm for 60 min, and (d) reconstituted spray-dried mAb B formulation after centrifugation at 10,000 rpm for 60 min. Spectra comparison of control and stressed samples showing Amide I (1600–1700 cm−1) and Amide II (1510–1580 cm−1) FTIR bands: (e) mAb A2 formulation, (f) mAb B formulation, and (g) mAb C formulation (control; supernatant; pellet from mixed samples; pellet from spray-dried samples).
Structural Changes of the Mixed or Spray-Dried mAb, as Indicated by the Amount of β-Sheets Measured Using FTIR
4. Conclusions
Stress mechanisms associated with the grinding action between two solid surfaces (impeller/drive unit as well as piston pump couplings) were successfully assessed using various stress models. Common stress types, such as air–liquid interfacial stress and shear stress, were readily ruled out. Cavitation, often cited as a potential stress type during mixing, was proven to be absent upon grinding in these two coupling systems. The fact that cavitation-induced stress and grinding led to different protein degradation pathways also suggested that cavitation is an unlikely stress mechanism for grinding. Heterogeneous and homogeneous nucleation of protein aggregation could not be confirmed as contributing to SvP formation in this study. Grinding-induced thermal stress matched well with the spray-drying model and was considered a feasible stress mechanism. This study offered an overall conclusion that processing equipment containing grinding solid surfaces accessible to mAb drug substance solutions can readily cause SvP formation due to grinding-induced thermal stress.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank Roche colleagues Miguel Saggu for his valuable input to SvP analysis, Kai Zheng for his guidance on FTIR use and application, and Sreedhara Alavattam, Adeline Boillon, Yilma Adem, and Vikas Sharma for helpful discussions.
- © PDA, Inc. 2018