Abstract
The BioPhorum Development Group Viral Clearance Workstream performed a collaborative retrospective analysis to evaluate packed bed chromatographic resin performance after repeated cycling for two commonly used chromatography steps in biopharmaceutical manufacturing: protein A and anion exchange. Key variables evaluated in the assessment included virus type, resin type, number of reuse cycles, and virus challenge. In this retrospective analysis of viral clearance data on naïve versus cycled resin, powered by the availability of a decade’s worth of accumulated industry data, clearance capability was not negatively impacted by resin cycling. This finding is consistent with publications showing that surrogates for viral clearance capabilities could be employed in lieu of testing the viral clearance of cycled resins for protein A and anion exchange chromatography. The rigorous analysis of the retrospective data supports the view that viral clearance studies for cycled resins are not necessary provided that appropriate cleaning methods are applied during repeated use of the chromatography columns.
LAY ABSTRACT: The manufacturing processes for biopharmaceutical products often include reusable chromatographic resins that remove process- and product-related impurities as well as potential contaminating viruses. Typically, chromatography resin is “cycled” through repeated steps of resin conditioning, product purification, and resin cleaning. The cycling approach has been evaluated in both small- and full-scale studies that show the performance parameters are maintained. The ability to remove virus is demonstrated separately in a focused small-scale virus-spiking study that is resource-intensive and costly. This paper is a retrospective review of industry data comparing virus removal by naïve and repeatedly cycled resins that summarizes the viral clearance impact of re-using protein A and anion exchange chromatography resins. The key variables evaluated in the assessment included virus type, resin type, number of cycles, and virus challenge. In this retrospective analysis, it was found that the viral clearance capability is not negatively impacted by resin cycling. This finding is consistent with other publications and supports the view that viral clearance studies for cycled resins are not necessary if appropriate cleaning methods are applied during the repeated use of the chromatography columns.
Abbreviations: AAV-2, Adeno-associated virus; A-MuLV, Amphotropic murine leukemia virus; AEX, Anion-exchange chromatography; B/E, Bind and elute; BVDV, Bovine viral diarrhea virus; C.P.G., Controlled pore glass; DEAE, Diethylaminoethanol; EMCV, Encephalomyocarditis virus; FT, Flow through; HAV, Hepatitis A virus; HSV-1, Herpes simplex virus type 1; LOD, Limit of detection; LOQ, Limit of quantification; LRF, Log10 reduction factor; mAb, Monoclonal antibody; MVM, Minute virus of mice; NaOH, Sodium hydroxide; PA, Protein A; PPV, Porcine parvovirus; QA, Quaternary amine; QP, Quaternized polyethyleneimine; qPCR, Quantitative polymerase chain reaction; Reo3, Reovirus type 3; SuHV-1, Suid herpesvirus; SV40, Simian virus 40; X-MuLV, Xenotropic murine leukemia virus
Background
Viral safety is one of several measures in place to ensure the safety of biotechnology products derived from mammalian cell lines and intended for therapeutic use. Rodent cell lines may contain endogenous retroviruses or retrovirus-like particles as measured by transmission electron microscopy (1). Furthermore, cell cultures may become contaminated by adventitious viruses introduced through raw materials or handling (2, 3). To contribute to the safety of biopharmaceuticals, purification processes include multiple orthogonal virus reduction steps, and there are regulatory requirements to demonstrate the ability of the purification process to remove or inactivate a wide variety of potential viral contaminants in investigational and commercial products (4⇓⇓–7).
Chromatography steps contribute to the cumulative viral clearance claimed for many biopharmaceutical purification processes. Chromatography resins are routinely reused for many discrete batches produced over months or even years while performance and product quality are maintained with an appropriate resin cleaning regime. For chromatography steps that are included in viral clearance claims, the impact of “repeated use” must be understood (7). The naming convention for chromatography resin reuse differs across firms, but this study uses “cycled resin” to denote chromatography resin that has been exposed to repeated cycles of use with exposure to product and to process solutions including cleaning solutions. The age of the resin, with respect to the resin manufacturing date, is not considered in this retrospective evaluation. Naïve resin refers to resin that has not been exposed to virus and has been exposed to few or no product cycles.
Firms have addressed the regulatory guidance by comparing the viral clearance for model viruses on naïve and cycled resin for individual products and processes. Cycled resin viral clearance studies are resource-intensive and costly. Generating resin that has been exposed to sufficient repeated cycles to simulate a resin lifetime may take 1 to 6 months and typically requires hundreds of grams of recombinant protein even at a laboratory bench scale. Viral clearance studies with naïve resin are often performed early in product development to support clinical trial applications, whereas studies on cycled resin are often performed months or years later to support marketing applications. Resin-cycling studies often do not include a contemporaneous comparison of naïve and cycled resin because that requires costly reevaluation of naïve resin in parallel with the evaluation of cycled resin. Within this analysis, evaluations of naïve and cycled resins that occur at different times (that is, not in parallel) have been identified as “nonconcurrent,” as this study design may add variability (e.g., virus stock purity, virus stock titer, virus assay conditions) that can confound the comparison of naïve and cycled resin.
Extensive industry data regarding the impact of chromatography resin cycling on viral clearance exists but not in an accessible, collated format. Individual firms often have experience with fewer products with limited variability in process conditions. Regulatory agencies have access to cycled resin viral clearance data submitted with marketing applications but often with limited virus study information. Contract laboratories conducting viral clearance studies have data from a variety of clients’ studies but often have limited process information. Some published reports have indicated that chromatography resins that have not shown performance decline for other attributes with cycling also do not show a decline in viral clearance. This has been reported for protein A chromatography and anion-exchange chromatography (AEX) resins, and additional experience with multiple chromatography resins has been reported by individual firms (8⇓⇓⇓⇓⇓–14).
The retrospective analyses presented here result from a rigorous statistical analysis of a large data set compiled by multiple individual firms. Data from studies representing real-world variation in product type, molecule isoelectric point, load impurity profile, resin ligand and back bone, surface chemistry, resin loading, and resin cleaning, as well as virus challenge, have been evaluated. Data were collected over multiple decades. This analysis contributes to an evidence-based discussion of whether cycling of resins has a detrimental impact on the viral clearance.
Materials and Methods
Data Source
A database was constructed using blinded survey responses from 12 biotechnology companies including AbbVie Bioresearch Center (Worcester, MA), Alexion Pharmaceuticals, Inc. (Boston, MA), Biogen (Research Triangle Park, NC), Bristol-Myers Squibb (Devens MA), GlaxoSmithKline plc (King of Prussia, PA), GlaxoSmithKline plc (Rockville, MD), ImmunoGen, Inc. (Waltham, MA), Merck & Co., Inc., Kenilworth, NJ, USA, Regeneron Pharmaceuticals Inc. (Tarrytown, NY), Genentech, Inc. (South San Francisco, CA), Shire Plc (Lexington, MA), Takeda Pharmaceuticals International Co. (Cambridge, MA), and UCB (Brussels, Belgium). Protein A and anion-exchange chromatography steps were surveyed to provide a large data set of viral clearance for commonly used manufacturing steps. Conversely, implementation of other chromatographic technologies such as cation exchange, hydrophobic interaction, and mixed-mode chromatography differ widely among firms and were not included in this analysis.
Firms contributed retrospective GLP-compliant viral clearance data spanning decades. The database was comprised of “paired observations”, where firms reported the clearance for both naïve and cycled resins for a given virus. Virus log10 reduction factor (LRF) was calculated and reported by contributors as described in eq 1.
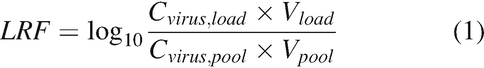 (1)Where Cvirus, load is the virus concentration in the load, Cvirus, pool is the virus concentration in the pool, Vload is the load volume, and Vpool is the pool volume.
(1)Where Cvirus, load is the virus concentration in the load, Cvirus, pool is the virus concentration in the pool, Vload is the load volume, and Vpool is the pool volume.
The firms indicated whether the naïve and the cycled resins were tested concurrently or nonconcurrently as described previously. Data were a representative sample of viral clearance studies performed to support product licensure but did not include all historical data generated by the 12 firms. The database did not capture the impact of resin cycling on process performance except for the ability to remove model viruses. No results were excluded owing to the reduced performance of the cycled resin compared to the naïve resin, and the data are consistent with the published capabilities of these manufacturing steps (15).
The results were categorized as “mAb” for monoclonal antibody-based processes and “non-mAb” for all other recombinant proteins to assess class effects. Recombinant protein, product, and molecule are all used to refer to mAb and non-mAb as appropriate. Model viruses were reported, although the strain and preparation method were not in the scope of this analysis. The clean-in-place (CIP) solution was reported for the AEX resins. Process parameters such as column loading and CIP solution contact time were disclosed for some, but not all, paired observations based on the disclosure policy of the firms.
Analysis
Data were compiled and blinded by the BioPhorum Development Group Viral Clearance Workstream facilitator and imported into SAS JMP v11.1.1 for statistical analyses. Results were filtered using two rules to provide a representative data set (Table I). Rule 1 assured that the mechanism of viral clearance on protein A was physical removal rather than inactivation during putative low pH elution (16). Rule 2 assured that the virus challenge was within ±1.0 log10 for the spiking studies of naïve and cycled resin comprising a paired observation, as this was considered a comparable evaluation based on cumulative experimental and assay variability (8). This limitation was necessary because the virus challenge in the load may influence the demonstrated virus removal by the step either by overloading of the resin, underloading of the resin, or the condition in which the LRF is limited by the limit of quantification (LOQ) of the assay.
Rules Established to Allow Direct Comparison of Viral Clearance by Naïve and Cycled Resins
Change in performance after repeated use was reported as the difference in LRF within a paired observation. Zero difference indicates that there is no difference in virus removal between the cycled resin and the naïve resin. A positive difference indicates that the cycled resin removes more virus than the naïve resin. Alternatively, a negative difference shows that the cycled resin removes less virus than the naïve resin. A change <1 log10 was not considered practically meaningful as implied by the published guidance describing a reduction in virus titer on the order of 1 log10 as negligible (7).
It was hypothesized that effective removal by the naïve resin may correlate with robust performance after repeated cycling. As such, the clearance ability for the naïve resin was categorized as:
<1.0 LRF: Step does not provide reliable removal of the model virus.
1.0 LRF ≤ x < 4.0 LRF: Step contributes to virus removal although the mechanism does not provide effective clearance.
≥4.0 LRF: Step is considered effective for virus removal, and the mechanism is robust.
Results
Protein A Chromatography Resin
Description of Protein A Paired Observations:
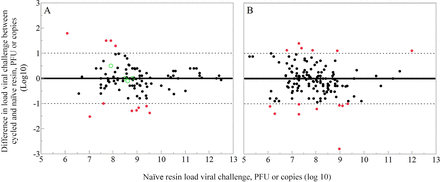
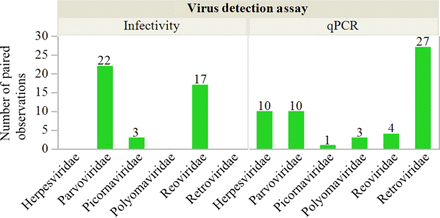
The submitted, blinded, and compiled results included 131 paired observations comparing viral clearance by naïve and cycled resin for protein A chromatography. The ability of the protein A resin to remove virus was determined by each company’s standard practices. Six virus families were represented, and the data set included enveloped and nonenveloped viruses. The data set was reduced by the filter rules in Table I to prevent assessment of enveloped virus inactivation and to ensure a similar virus challenge among paired observations. Figure 1A illustrates the application of Rules 1 and 2. Four paired observations were excluded owing to the use of the infectivity assay with enveloped virus, whereas 11 paired observations were excluded because the virus challenge in the naïve and the cycled resin differed by >1.0 log10. As the virus challenge was not reported for a further 19 paired observations, they could not be confirmed to conform to Rule 2; therefore, they were excluded from the analysis. After the filter rules described in Table I were applied, a total of 97 paired observations remained. The remaining data represented protein A processes for sixteen mAbs and eight non-mAbs. Figure 2 shows that the model virus families included in the analysis offer diverse physicochemical properties as described in Table II.
Application of filter rules for protein A chromatography and AEX. (A) Protein A chromatography and (B) AEX. Positive numbers correspond to increased load viral challenge in the cycled resin compared to the naïve resin. Green (○) denotes paired observations excluded owing to Rule 1. Red denotes paired observations excluded owing to Rule 2. Black denotes paired observations included in the analysis.
Number of paired observations included for each virus family tested with cell-based infectivity assay and qPCR assays for protein A chromatography. Herpesviridae and retroviridae clearances evaluated by the infectivity assay were not included owing to the potential inactivation of the enveloped virus by the putative low pH elution.
Model Viruses Used in Spiking Studies
The resins were classified by their base matrix and caustic stability, including: caustic stable agarose, noncaustic stable agarose, and noncaustic stable controlled pore glass (C.P.G.). The source of the protein A ligand was not considered in this analysis. Most of the observations in this analysis are from agarose resin that is not stable to caustic solutions.
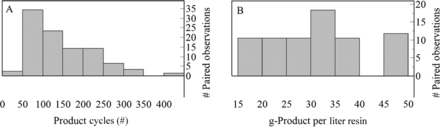
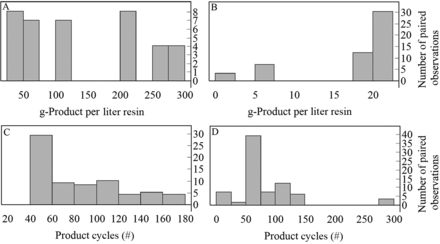
The selected process parameters included the number of product cycles and the product loading (gram product per liter resin). Figure 3 shows the distribution of product cycles and the product loading for the paired observations. The detailed process conditions were beyond the scope of this study; however, the processes from the 12 biotechnology firms include a breadth and diversity of buffer matrices, phase durations, and wash strategies.
Number of product cycles and product loading for paired observations of protein A chromatography. (A) Distribution of resin cycles and (B) grams of product per liter resin loaded for protein A paired observations. Note: column loading was not reported for all paired observations.
Viral Clearance by Protein A:
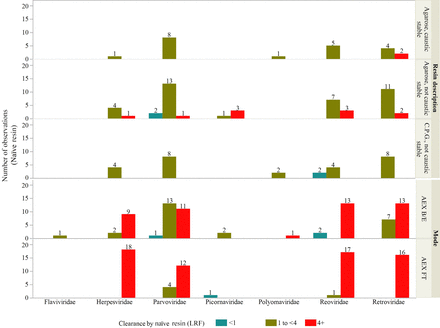
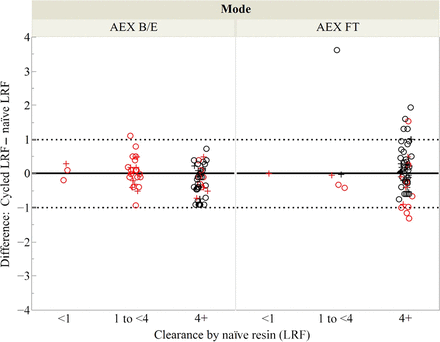
The variation observed in this data set is consistent with previously published literature in which the LRF by Protein A is typically between 1 LRF and 4 LRF, but on some occasions, there is <1 LRF or >4 LRF (15⇓⇓–18). Figure 4 shows that it was most common for the naïve protein A resin to have between 1 and 4 LRF across the six virus families and the resin physical properties.
Naïve protein A and AEX resin LRFs by resin type or mode and virus type.
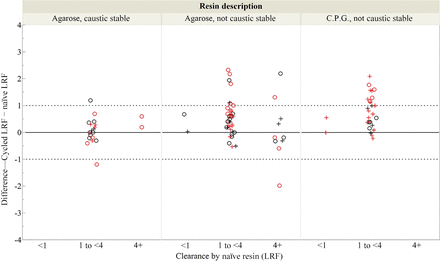
Figure 5 compares the change in LRF for paired observations by resin type for all virus families. Table III summarizes the comparison of the clearance within paired observations by resin type within the context of concurrent or nonconcurrent testing. For the agarose resin that is caustic stable, the change in the LRF between the cycled and the naïve resin is within the generally accepted experimental variability of 1.0 LRF. For the two observations exceeding the 1.0 LRF difference, one demonstrated increased clearance and one demonstrated decreased clearance. Both were tested nonconcurrently. For the agarose resin that is not caustic stable, the cycled resin LRF is either comparable to or better than the naïve resin for 47 of the 48 paired observations. For the C.P.G. resin that is not caustic stable, the cycled resin LRF is either comparable to or better than the naïve resin for all 28 of the paired observations. Within this data set, performance changes exceeding 1.0 LRF occurred disproportionately more often when paired observations were tested non-concurrently rather than concurrently. Among protein A examples where the difference in the clearance between the naïve and the cycled resin was >1 LRF, 16 of 20 relied upon a qPCR assay. This suggests greater variability either for enveloped viruses or for the qPCR assay, as the enveloped virus quantitation was collinear with qPCR in this data set. As shown in Figure 5, the LRF for the cycled resin was generally comparable to or better than the LRF of the naïve resin, even when the clearance mechanism was not “effective” (i.e., <4.0 LRF).
Change in clearance over protein A resin lifetime as a function of resin type and clearance by naïve resin. Symbol (○) denotes the naïve and the cycled resins tested nonconcurrently. Symbol (+) denotes the naïve and the cycled resins tested concurrently. Red denotes qPCR-based assay and black denotes infectivity assay.
Summary of the Viral Clearance in the Paired Observations Using Concurrent or Non-Concurrent Testing Approaches
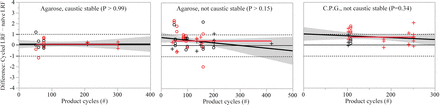
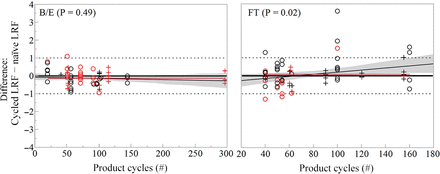
The effect of the product cycles and the total product loading on the change in virus removal was assessed statistically at 0.05 significance level. Figure 6 shows the number of product cycles does not have a significant effect on the change in viral clearance over the resin lifetime. The lack of effect is illustrated by the shaded 95% confidence region of the fitted line that encompasses the horizontal line representing the mean change in the LRF. Not all firms were permitted to disclose the gram product per liter resin required to calculate the total product loading, so a subset of the data was analyzed and demonstrated no significant effect on the difference in clearance achieved by the cycled and the naïve resin (data not shown).
Change in clearance over resin lifetime as a function of the number of cycles for protein A. Shaded gray is the 95% confidence region for the linear fit, whereas the red line is the mean. Symbol (○) denotes the naïve and the cycled resins tested nonconcurrently. Symbol (+) denotes the naïve and the cycled resins tested concurrently. Red denotes qPCR-based assay and black denotes infectivity assay.
AEX Resins
Description of AEX Paired Observations:
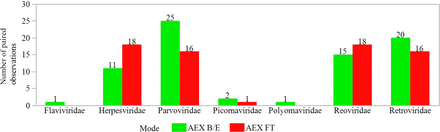
The submitted, blinded, and compiled results included 166 paired observations comparing the viral clearance by naïve and cycled resins for AEX. The AEX processes were described as operating in flow-through (FT) mode or bind/elute (B/E) mode. This description corresponds to the state of the recombinant protein, which either flows through the packed bed during the load (FT) or binds and is subsequently eluted (B/E). The ability of AEX to remove virus was determined by each company’s standard practices, and the data set was reduced by a filter rule to ensure similar virus challenge among paired observations. Figure 1B shows application of Rule 2 for AEX. Fifteen paired observations were excluded because the virus challenge in the naïve and the cycled resin differed by >1.0 log10, whereas seven paired observations were excluded because the virus challenge was not reported. After applying the filter rules described in Table I, a total of 144 paired observations remained. The remaining data represented AEX processes for 17 mAbs and 11 non-mAbs. Figure 7 shows the distribution of the model virus families included for the B/E and the FT modes. Flaviviridae and polyomaviridae were evaluated exclusively with the B/E processes. A relatively similar number of paired observations for the B/E mode (75) and the FT mode (69) were reported.
Number of paired observations reported for each virus family tested with B/E or FT AEX.
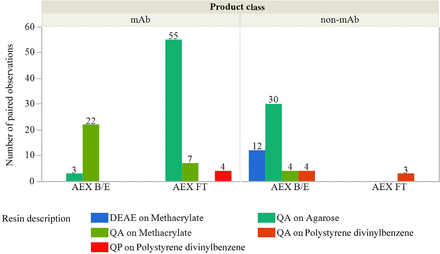
The physical properties of the resin included the combination of the resin backbone (i.e., agarose, methacrylate, or polystyrene divinylbenzene) and the functional group (i.e., diethylaminoethyl or quaternary amine) are shown in Figure 8. For mAbs, the AEX resin was generally operated in FT mode using a strong anion-exchange (i.e., quaternary amine [QA]) ligand on varying backbones. Non-mAb AEX was generally operated in B/E mode, using a variety of combinations of ligands and backbones.
Number of paired observations reported for AEX backbone, functional group, mode of operation, and product class.
The selected process parameters included the number of product cycles and the product loading (gram product per liter packed resin). Figure 9 shows the column loading and the number of product cycles for both the B/E and the FT AEX. When comparing the two modes, the B/E AEX features 2- to 10-fold lower product loading than the FT, although both are routinely cycled 50–150 times. The AEX operating conditions are typically dependent on the recombinant protein isoelectric point, and the AEX data set included products with a broad distribution of isoelectric points ranging from 4.2 to 9.5. The detailed process conditions were beyond the scope of this study; however, the processes from the 12 biotechnology firms include a breadth and diversity of buffer matrices, pH, conductivity, phase durations, and product collection strategies.
Product loading and the number of resin product cycles for paired observations for AEX. Column loading for FT (A) and B/E (B) and the number of resin cycles for FT (C) and B/E (D). Note: column loading was not reported for all paired observations.
Viral Clearance by AEX:
The performance by the naïve resin in this data set is shown in Figure 4. Viral clearance was consistent with previously published data in which the LRF is typically >4 log10 for the FT mode whereas the B/E mode was more dependent on the virus type (15). Figure 10 compares the change in the LRF for the paired observations for AEX in the B/E and the FT modes according to the previously described categories. The dashed lines represent a change of 1.0 log10, which is the generally accepted experimental variability. Table III summarizes the comparison of the paired observations within the context of concurrent or nonconcurrent testing. Regardless of the performance of the naïve resin and the mode of operation for AEX, the paired observations typically provided clearance within 1 LRF (134 of 144 cases). For AEX resin operated in the B/E mode, the only instance where the difference exceeded one LRF was an increase in clearance capability. For AEX operated in the FT mode, the variation in LRF was within 1.0 log10 in 60 of 69 of the paired observations, and there are two cases where a decrease in clearance exceeded 1.0 log10. In these two cases, the reduction in the clearance exceeding 1.0 LRF correlates with nonconcurrent testing. When the cycled AEX resin clearance was not within 1 LRF of the naïve resin, 90% (9 of 10 cases) were tested nonconcurrently.
Change in clearance over AEX resin lifetime as a function of the operating mode and clearance by naïve resin. Symbol (○) denotes the naïve and the cycled resins tested nonconcurrently. Symbol (+) denotes the naïve and the cycled resins tested concurrently. Red denotes the residual virus detected when testing the cycled resin, whereas black denotes the virus reduced below assay detection limits when testing the cycled resin.
So far contributing firms have not seen product contacting cycles or total mass loading impact the ability of the resin to remove virus over the resin lifetime whether AEX is operated in the FT or the B/E mode. The team evaluated the hypothesis that the number of cycles has no impact on the change in clearance over the resin lifetime at 0.05 significance level, as shown in Figure 11. For B/E, there is no significant change in the clearance as a function of product cycles. There is a statistically significant increase in the clearance as a function of product cycles for the FT AEX, although the apparent increase relies on instances where the virus was reduced below the assay detection limit in the cycled resin. The increased clearance in 5 of these 6 cases may reflect a change in the spiking study design (e.g., assay detection limit or load virus titer) associated with the nonconcurrent testing of the cycled and the naïve resin rather than a change in the resin performance. As was the case for protein A, it was not permissible for all firms to contribute detailed process information such as column loading. The available data were evaluated, and the total product loading had no effect on the difference in the clearance achieved by the cycled and the naïve resin at 0.05 significance level (data not shown).
Change in clearance over resin lifetime as a function of the number of cycles for the AEX. Light shaded gray denotes the 95% confidence region of the linear fit, whereas the red line is the mean. Symbol (○) denotes the naïve and the cycled resins tested nonconcurrently. Symbol (+) denotes the naïve and the cycled resins tested concurrently. Red denotes the residual virus detected when testing the cycled resin, whereas black denotes the virus reduced below assay detection limits when testing the cycled resin.
CIP Procedures
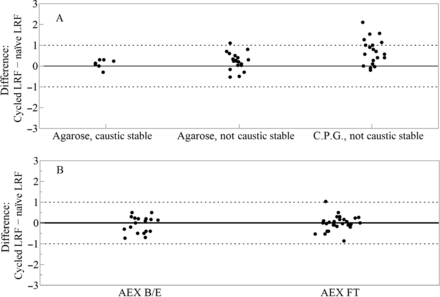
The impact of the harsh resin cleaning regimens after repeated cycles was evaluated for the protein A and AEX resins based upon the vendor indication that the functionality may potentially be lost. This potential was assessed via the extensive data set contributed by each company using their standard practices. Protein A resins compatible with sodium hydroxide cleaning showed no change in performance over the lifetime on average (Figure 5). Protein A resins that do not tolerate caustic cleaning showed increased clearance for cycled resin exceeding 1.0 log10 for cycled resin in 17 out of 76 paired observations.
For the AEX resin, sodium hydroxide was used during CIP for all paired observations, and the concentration was as high as 1.0 M NaOH. The mean change in clearance was determined for the seven categories of cleaning solutions used for the B/E and the FT mode. categories included:
0.1–0.5 M sodium hydroxide with contact time either non-disclosed, <1 h, or ≥1 h (3 categories).
1.0 M sodium hydroxide with contact time either non-disclosed or ≥1 h (2 categories).
Sodium hydroxide combined with sodium chloride with contact time either <1 h, or ≥1 h (2 categories).
The difference in the mean LRF was calculated for all pairs of cleaning solutions and ranged from 0.00 LRF to 0.66 LRF for the B/E and the FT. The differences in means is less than the practical experimental variation of 1 LRF associated with the studies. As such, comparison of column CIP procedures encompassing a variety of sodium hydroxide strengths and contact times do not support the view that the resin functionality to remove virus is lost because of harsh cleaning with these solutions. This retrospective review concludes that the cleaning procedures listed above are suitable for the wide variety of anion-exchange functional groups and resin backbones shown in Figure 8.
Timing of the Evaluation for the Naïve and the Cycled Resins
Many sources of variability are not controlled when the naïve and the cycled resins are tested nonconcurrently, with examples including: virus titer, virus lot, virus preparation impurity level, and assay detection limit. Many of these factors tend to be held constant when the naïve and the cycled resin are tested concurrently. For protein A, 51 out of 97 paired observations were evaluated nonconcurrently. For AEX, 99 out of 144 paired observations were evaluated nonconcurrently. Figure 12 demonstrates the reduced variability when the database was limited to concurrent evaluations of paired observations. Notably, this limited data set of concurrent tests includes no cases where the clearance is reduced by >1.0 LRF in the cycled resin. Several case studies illustrate the potential variability from the nonconcurrent testing.
Change in viral clearance during concurrent evaluation of the naïve and the cycled chromatography resins. Variability is reduced for protein A (A) and AEX (B) when paired observations are evaluated concurrently.
For caustic stable protein A, there is a single case where the clearance is reduced by >1.0 LRF (Figure 5). In this case, paired observations were evaluated nonconcurrently. Retrovirus clearance was measured by qPCR and was 1.2 LRF lower in the cycled resin than in the naïve resin. Parvovirus and reovirus clearances were within 0.5 LRF in the paired observations, suggesting that the change in the clearance was not related to a change in the integrity of the resin.
For noncaustic stable agarose protein A, there is a negative difference exceeding 1.0 LRF for the cycled resin in one case out of 48. The decreased LRF was observed for a single virus type (herpesviridae) measured by qPCR, whereas the clearances for four other virus types tested with the same product and cycled chromatography resin were either within the accepted assay variation or increased. The clearance of herpesviridae by the naïve resin was 5.4 LRF, which exceeds the typical removal observed by protein A chromatography, but there was no justification to invalidate the result (15). In this case, the paired observations were evaluated nonconcurrently and there were many factors that were not held constant.
For the FT AEX, the two cases where clearance by the cycled resin was >1.0 LRF lower than the clearance by the naïve resin corresponded to nonconcurrent testing (Figure 10). One case evaluated herpes virus (1.16 LRF decrease in clearance for the cycled resin) and the other case evaluated parvovirus (1.30 LRF decrease in clearance for the cycled resin). These were processes for different mAbs; one process used a strong ion exchanger on an agarose backbone, whereas the other used a strong ion exchanger on a polystyrene divinylbenzene backbone.
In one example for the FT AEX, the potential variability associated with the nonconcurrent testing corresponded to an unusual increase in the clearance by the cycled resin. In this case there appears to be a 3 LRF increase in clearance by the cycled FT AEX resin compared to the naïve resin. In this single observation, the cycled resin demonstrated a 7.5 LRF for minute virus of mice (MVM), whereas the naïve resin achieved a 3.98 LRF. Evaluation of four other viruses with the same product and process resulted in an LRF within 1.0 log10 for the naïve and the cycled resin.
Discussion
A virus-spiking study is a model for endogenous retrovirus-like particles and/or a contamination event in manufacturing and is used to assess the viral clearance achieved by the purification process and to assure the virus safety of the product. Viral clearance is assessed by evaluating the amount of “spiked” model virus present in the load material and comparing it to the amount of model virus present in the product pool (eq 1), resulting in an LRF for that step. Reported LRF for spiking studies conducted in this manner can be impacted by the total virus challenge in the load material. For example, a high virus challenge in the protein A chromatography load may result in increased virus removal because the majority of the virus flows through the protein A resin, while a small, putatively constant quantity co-purifies with the product (11, 16). Because the AEX often reduces the virus to nondetectable levels in the product pool, increased virus challenge may proportionately increase the demonstrated LRF for this step as shown in eq 1 (15). In these examples, it is not the chromatography that is impacting the LRF, but rather the experimental variability of the virus-spiking study. Hence, Rule 2 was incorporated in the analysis of the data sets (reference Table I). These examples are especially likely to occur when the replicate runs are not conducted at the same time or under the same protocol, which occurs with some regularity for the naïve and the cycled resin. The resulting LRF from a virus-spiking study may be limited by the total virus challenge and is not necessarily a demonstration of the chromatography step’s maximum ability to remove virus nor does it reflect that either the naïve or the cycled resin is better or worse at removing virus.
Protein A chromatography resin is specifically designed to primarily bind the Fc portion of mAbs or the Fc-fusion proteins, while impurities such as virus are separated. Fc-containing products bind with high specificity to the protein A chromatography functional group during the load phase and most virus remains unbound. A portion of the virus associates nonspecifically with the resin backbone, the resin ligand, or the Fc-containing product and may co-elute with product during the elution phase, which is commonly acidic (18). Physical removal of virus by protein A chromatography has been reported to be highly consistent for a given product but varying widely across products, suggesting that both virus–resin and virus–product interactions are responsible for virus adsorption and co-elution with the product (16⇓–18). When considering mechanisms for potential retention of virus during the load phase, interactions between the virus and the cycled resin may decrease as binding sites are occupied or partially denatured. This retrospective analysis showed that in real-world applications, protein A chromatography was robust for viral clearance, in that it removed virus to the same level whether a naïve resin or a cycled resin was used. For protein A resins that are cleaned with sodium hydroxide, the difference in the LRF between new and used resins was within 1.0 log10 in almost all observations (19 of 21). For protein A resins that are not caustic stable (agarose or C.P.G.), the difference in the clearance between the new and cycled resins was within 1 log10 in 58 of 76 cases. When paired observations of noncaustic stable resin were not within ±1 LRF, the clearance by the cycled resin exceeded the clearance by the naïve resin in 17 of 18 cases. A majority of these cases measured virus using qPCR (15 of 18). Overall, there are only 2 of 97 cases where the cycling protein A resin decreases the clearance by >1 LRF compared to the naïve resin.
A systematic increase in the LRF was observed for protein A, especially for resins that cannot be cleaned-in-place with sodium hydroxide. For protein A resins that are repeatedly cycled, potential changes to the ligand function, the ligand density, the ligand accessibility, or the base matrix may occur and result in increased virus flow-through during the load phase, thus increasing the viral clearance by the cycled resin. One potential mechanism to explain the increased clearance by the cycled protein A resin is the blocking of nonspecific binding sites that may exist in the naïve resin.
The AEX resin is typically positively charged over a wide range of pH. As such, a recombinant protein will either bind to the resin or flow through, depending on protein isoelectric point. In either case, the separation may be designed such that impurities, including viruses, are separated from the product based on differences in electrostatic interactions with the positively charged functional group (19). AEX viral clearance has been reported to be highly consistent across products when ionic interactions are maintained (i.e., same chromatography resin, conductivity and salt composition), further supporting the view that separation is driven by interactions between the virus and the functional group rather than by virus–product interactions (20). The analysis of paired observations in this data set was intended to show if the functional group degrades or becomes occupied after repeated cycling, and the analysis suggests that has not occurred. For the AEX resins operated in the B/E mode, 74 of 75 paired observations were within 1.0 LRF. For the AEX resins operated in the FT mode, 60 of 69 of paired observations were within 1.0 log10. Overall, 142 of 144 paired observations demonstrated no notable negative impact of cycling on AEX. Repeated use of AEX resins had no negative impact on viral clearance capabilities when appropriate cleaning techniques were applied, despite the marked differences in the feed streams, the functional group, the resin backbone, and the cleaning regimen of the diverse data set.
Inherent to the spiking study is the laboratory procedures governing virus stock purity, titer, and assay intermediate precision. It was not feasible to account for this variability in our retrospective analysis. Our analysis identified paired observations in which clearance by the new and the cycled resins differed by >1.0 log10. The vast majority of these observations were associated with nonconcurrent evaluation of new and cycled resins and hence the measured difference in the LRF cannot necessarily be attributed to the impact of resin cycling. In contrast, when concurrent evaluation of new and cycled resins was employed, the difference in the LRF between the new and the cycled resins was <1 LRF or not practically significant (reference Figure 12). Continued efforts to reduce the assay and the virus preparation variability are critical to reduce the overall variability in spiking studies (21, 22).
Conclusion and Recommendations
A retrospective analysis of model viral clearance from 12 biotechnology firms spanning decades has shown that viral clearance capability of protein A and AEX is not negatively impacted by resin cycling independent of the virus type, the resin type, the number of cycles, the virus challenge, and the molecule isoelectric point (range 4.2–9.5). The difference in the viral clearance between the naïve resin and the cycled resin was typically no greater than the commonly accepted experimental variability of 1.0 log10. The variability of the viral clearance test reagents (for example, virus stock) and associated analytics (e.g., LOQ, precision) needs to be considered when assigning causality to a given parameter as these factors can be significant. The data show a substantial source of variation is nonconcurrent evaluation of the naïve and the cycled resins, which may confound lifetime effects with effects from the virus titer, the virus purity, or the assay. Another source of variation for protein A chromatography is virus assay by qPCR, which may be because of less mature qPCR practices than are the current standard in industry, as this extensive data set represents data collected over many years. If firms perform a virus-spiking study to determine that virus clearing ability is maintained throughout the lifetime, the BioPhorum Development Group Viral Clearance Workstream recommends:
The evaluation of the naïve and the cycled resins with appropriate controls (e.g., virus titer, purity, ratio of infectious to noninfectious virus, and assay), which may be accomplished by testing the naïve and the cycled resins concurrently for the ability to remove model viruses.
The use of well-designed qPCR assays to reduce the variability of the determined LRF versus infectivity assays (21).
The findings of the retrospective review are consistent with publications showing that product yield, product breakthrough, and column pressure provide indicators of resin degradation that can be monitored in lieu of testing virus removal by cycled protein A chromatography resin (9⇓–11). A similar approach has been suggested for AEX, where in-process monitoring of DNA or host cell protein removal has provided demonstration of maintained performance in place of direct evaluation of viral clearance by cycled resin (13, 14). The BioPhorum Development Group Viral Clearance Workstream’s rigorous analysis of the retrospective data supports the view that viral clearance studies for cycled resins are not necessary, if appropriate cleaning methods are applied during the repeated use of chromatography columns.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgements
This article describes a consensus view from the BioPhorum Development Group Viral Clearance Workstream. The authors sincerely thank the members of the team for their contributions at monthly BPDG discussions and in the preparation of this manuscript.
Since its inception in 2004, the BioPhorum has become a trusted environment where senior leaders of the biopharma industry come together to openly share and discuss the emerging trends and challenges facing their industry. BioPhorum currently comprises 71 manufacturers and suppliers deploying their top 2000 leaders and subject matter experts in seven Phorums: Drug Substance, The Development Group, Fill Finish, The Technology Roadmap, BioPhorum IT Group, BioPhorum Supply Partners, and BioPhorum Cell and Gene Therapy. The Viral Clearance Workstream is part of the Biophorum Development Group.
This article is a composite view of opinions shared by the whole of the BPDG Viral Clearance Workstream and should not be attributed to the individual positions of the participating companies.
- © PDA, Inc. 2019