Abstract
Chemical indicators are commonly used in hospitals to monitor steam sterilization conditions, indicating that medical devices are safe to be used. The results are stored for future evidence in the event of an infection incident root cause analysis. This type of indicator is also becoming an option for cycle monitoring in pharmaceutical steam sterilizers, improving cycle control. They are constructed and tested according to published standards, but contradictory results between chemical indicators and cycle printouts have a critical impact on process control. We found that Type 6 chemical indicators used in steam sterilizer cycles did not perform according to their intended use, showing an “approved” result in a “failed” cycle (a false positive). This study demonstrated that Type 6 chemical indicator specifications are not adequate for monitoring steam sterilizers. A change in standards is therefore needed.
- Chemical indicators
- Cycle monitoring
- Process indicators
- Biological indicators
- Steam sterilization, Medical device
Introduction
Chemical indicators (CIs), especially Types 5 and 6, are designed to react to all steam sterilization cycle critical process variables, showing a pass result only if all stated conditions are met. In most hospital institutions around the world, they are the key instrument used to pass a sterilized medical device for clinical use (1). The performance of these indicators is guided by international standards and challenged using standardized equipment, known as a biological indicator Equipment resistometer (BIER) vessel (2, 3). CI Type 5 is an integrator, mimicking biological indicator performance, with its results integrated during the cycle (1). CI Type 6 is an emulator, which will only show a pass result when the specified temperature and time are achieved in the exposure phase (4). Based on these characteristics, it is expected that a CI Type 5 will integrate toward its pass area if the temperature and time are present in a cycle, and a CI Type 6 will only react during the exposure phase, showing a pass when total exposure time and temperature are reached (5).
Commercially available steam sterilizers have their cycles and performance specified according to the international standard ISO 17665. However, only the exposure phase is stipulated, leaving the conditioning and drying phases undefined, which allows wide variation in the duration of the come-up time in the conditioning phase (6). Reports from the field, where cycles have been canceled because of failures or intentionally, have raised questions about the effectiveness of these indicators, especially when, in qualified steam sterilizers, both Type 5 and Type 6 CIs reached the end point (pass) despite the exposure phase not being completed according to physical indicators (the temperature and time printout of the sterilizer).
Objectives
The study objective was to determine the effectiveness of those Type 5 and 6 CIs that have been certified as compliant with current international standards and to compare the results between chemical and physical indicators when applied to qualified steam sterilizers. The intent of this study was not to compare different CIs manufactured This has been referenced in many articles and an important one is referenced in this document (4, 5), which does the comparison. The results from this study, when added to other CI papers, will have a complete rationale that should support a revision of current standards.
Methods
Thermal qualification reports according to ISO 17665-1 of 95 steam sterilizers from different hospitals in Brazil were analyzed to determine the average common come-up ramps and a minimum of 3 min was determined. This time was programed into the BIER vessel cycle to simulate a real steam sterilization cycle come-up ramp and was compared with the standard 10 s come-up ramp. A Type 6 CI from the same manufacturer and in two different lots with a pass specification of 134°C and 4 min exposure was chosen, and a Type 5 CI from the same manufacturer and two different lots with a pass specification of 121°C and 22.9 min exposure, or 135°C and 1.9 min exposure, with results presented by a moving front window instead of a color change. Triplicate studies were conducted in a BIER vessel, with the temperature adjusted to 134°C, using three Type 5 CIs and three Type 6 CIs for each cycle configuration. Cycles 1, 2, and 3 were programed with a 10 s come-up ramp, and the exposure time was set to 2, 3, and 4 min respectively. Cycle 4 had the come-up ramp adjusted to 3 min by controlling the pressure increase over time, linear until reaching the exposure phase, which was set at 3 min. Cycle 5 was identical to cycle 4, but the exposure was reduced to 2 min. All cycles had a single vacuum pulse at the conditioning phase, according to the standard.
Results
The CIs performed according to stated values in all cycles with the 10 s come-up ramp. In Cycle 2, a difference was observed between the Type 5 and Type 6 CIs: Type 5 CIs showed a pass result because of the integration of the temperature and time from the start of the exposure phase, whereas Type 6 CIs showed a failed result because only the required temperature was reached, but the time was too short (Table I).
Total Type 5 and 6 Chemical Indicator Test Resultsa
In Cycles 4 and 5, with the 3 min come-up ramp (simulating an actual hospital sterilizer), with 3 min and 2 min exposures, respectively, at 134°C, Type 6 CIs should have shown failed results but instead passed with exposures below the end user labeled specification instructions of 134°C and 4 min exposure. Type 5 CIs, which had a stated value of 135°C for 1.9 min, all passed with a 3 min exposure but 2 out of 9 failed with a 2 min exposure.
Discussion
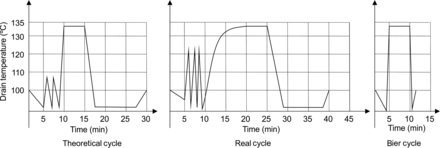
CIs are constructed according to ISO 11140-1 and have their efficiency tested in the BIER vessel, which only simulates the exposure phase of a sterilizer, not the real sterilization cycle. Also, steam sterilizer process validation standard ISO 17665 does not establish come-up ramp criteria, which allows qualified equipment to have come-up ramps in dry load cycles ranging from 3 to 12 min. For liquid sterilization, the come-up ramps can be even longer than the exposure phase, and CIs cannot be used. Comparing both equipment standards, an important difference was noticed in the conditioning phase. In the BIER vessel, a 10 s come-up time was needed, and for the steam sterilizer there was no performance requirement (Figure 1). CIs end point performances are only obtained in BIER vessels. With a 3 min come-up ramp, both CIs did not perform according to end user instructions for use specification, but Type 5, which is an integrator, could produce viable end point results at a maximum 3 min come-up ramp. Type 6 CIs were not adequate for come-up ramps longer than 10 s.
Cycle graphs from a theoretical sterilization cycle (ISO 17665), a real sterilizer cycle, and a BIER vessel cycle. The main difference is observed at the conditioning phase, where the come-up ramp, which is the time between the last vacuum point and the beginning of the exposure phase, is different in all graphs. This demonstrates that chemical indicators tested in a BIER vessel will not have the predicted performance in a real sterilizer.
The intent of this study was not to compare different CI makes as this has already been done (4, 5). The results from this study support a revision of CI standards ISO 11140-1 and ISO 18472 (addressing the BIER vessel cycle configuration), where the conditioning phase of real steam sterilizers is taken into consideration in the specification of CIs, and performance tests should solve the unexpected results observed in our study. This revision should also take into consideration the limitation of CI Type 6 color change interpretation, were a color reference to determine if a pass condition was obtained may vary between users, and the precise exposure time is hard to determine (4). To improve CI monitoring techniques, a revision of ISO 17665, addressing the impact of the come-up ramp duration on the CI results will help end users better understand how to correctly choose the indicator to monitor the sterilization cycle. Until the standards are revised, we recommend that thermal qualification technicians limit the come-up ramp to 3 min in their sterilization process qualification, allowing healthcare professionals to use a Type 5 CI (combined with biological and physical indicators results) to ensure adequate cycle monitoring, while observing local regulations and recommendations. The use of a Type 6 CI may cause the end user to clear a load for clinical use that was not processed according to medical device instructions with strict time and temperature requirements.
Conclusion
We do not advocate the complete elimination of Type 6 chemical indicators. Rather, the intent was to demonstrate that current standardized testing conditions do not accurately simulate the operations of a real sterilizer and that chemical indicators are not providing the result each manufacturer states in their instructions for use. Because resistometer cycles were developed for characterization of biological indicators, if configured correctly, and considering the chemical reaction characteristics, CIs may be used in qualified sterilizers. Our recommendation is achievable because a Type 6 CI with a 7 min exposure will be able to react correctly to cycle failures when used in a 4 min exposure. This evidence should help convince users and manufacturers that a revision of the standards and resistometer cycles is required.
Conflict of Interest Declaration
The authors declare that there are no known conflicts of interest associated with this publication and no competing interests exist.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
- © PDA, Inc. 2020