Abstract
A one-year study to establish the container closure integrity (CCI) performance landscape of systems comprising rubber stoppers and glass vials was performed. Focus was on addressing the issues of CCI performance versus: (a) time, (b) compression levels and residual seal force (RSF) values, and (c) potential variation in results based upon the deterministic measurement method (tracer gas and frequency modulated spectroscopy). To reduce sample size to a manageable number, the study was based upon a design of experiments that considered a range of: (a) stopper formulations, sizes, and configurations; (b) vial sizes, types, and suppliers; and (c) compression levels. All systems showed good performance; there was no decrease in CCI with time, highlighting the general robustness of rubber–glass vial systems. Lower compression/RSF values tended to give slightly lower performance and wider scatter in data and thus should not be recommended for commercial applications. A small amount of oxygen exchange through the stoppers was observed for all systems—to be expected because rubber is known to be gas permeable. Consistent with models, RSF values decreased initially and then remained constant. Results are consistent with the literature.
- Container closure integrity
- CCI
- Vial system
- Leak rate
- Residual seal force
- Tracer gas leak detection
- Frequency modulated spectroscopy
- Gas permeability
Introduction
Container closure integrity (CCI) performance is an essential feature of a drug product container system. CCI is demonstrated when a container system package meets its specific maximum allowable leak limit (MALL) (i.e., the smallest gap, or leak rate, that places drug product quality at risk)—established to maintain drug product quality attributes for sterility and physiochemical stability through expiry. There are many aspects of CCI; for example, it may prevent ingress of oxygen or carbon dioxide, or prevent loss of vacuum. Note that the MALL is specific to the drug product and container system. These points are discussed in detail in the recent USP guidance: General Chapter <1207> Package Integrity Evaluation—Sterile Products (1). This describes methods to evaluate CCI—strongly endorsing deterministic methods (e.g., tracer gas leak detection) where possible, vis-à-vis probabilistic methods (e.g., tracer liquids). DeGrazio has described a holistic, multistep process to assemble a vial-based container system that will provide CCI (2). Key in this holistic process is selection of the proper vial, rubber stopper, and seal system (based, inter alia, on proper interference fit and seal length), with the proper stopper compression level.
For container systems comprising rubber stoppers and glass vials, some key performance aspects have yet to be evaluated across a broad range, in particular as these aspects relate to stopper variations (formulation, configuration, and size) and vial variations (configuration, size, and supplier). These aspects are:
CCI performance as a function of time,
correlation of CCI performance with compression level and residual seal force (RSF) as a function of time, and
possible variation in CCI performance results from use of different deterministic methods.
The present study addresses these aspects.
Davidson and Mangus examined the systems given in Table I—at the initial time and with tracer gas (helium) leak detection (hereafter called He-leak) (3). Variations considered were:
Stopper Formulations: West Pharmaceutical Services, Inc. 4023/50 Gray (bromobutyl-based) and 4432/50 Gray (chlorobutyl-based).
Stopper Sizes: 13 mm; 20 mm.
Stopper Configurations: serum; lyophilization.
Vial Sizes: 2R; 6R.
Vial Types: Straightwall; European blowback (EU); US blowback (US).
Compression Levels: Low; Medium; High.
Combinations for Initial Time Study. Stoppers and seals were West Pharmaceutical Services, Inc. products.a Compression targets were low, medium, and high corresponding to approximately 10%–15%, 20%–35%, and 25%–40% for 20 mm stoppers and 20%–30%, 45%–55%, and 50%–60% for 13 mm stoppers, respectively. These corresponded to residual seal force values of 5–18 lbs.
A comparative frequently used for evaluation of He-leak data is that developed by Kirsch et al. (4). He-leak values were correlated with risk of microbial ingress for vials with holes of known diameter. Results are recapitulated in Table II. Note that a more recent report endorses the merit of the Kirsch et al. study (5).
Correlation of He-Leak Values to Risk of Microbial Failure per Kirsch et al.a
The He-leak values of Davidson and Mangus typically were <2 × 10–7 cm3/s for 20 mm stoppers and <7 × 10–8 cm3/s for 13 mm stoppers. These correspond to 0% risk of microbial ingress per Table II. Mathaes et al. examined a limited set of stoppers at varying compression levels and RSF values and obtained comparable results. At the initial time, all provided good CCI as determined by He-leak values < 10–7 millibar-liter/s (units of cm3/s can be reported as millibar-liter/s) (6, 7). These studies indicate an initial robustness of system performances across a broad range of stoppers and vials, but do not address the three key aspects noted above. It must be emphasized that for any drug product, CCI performance of the vial–stopper system must be such as to meet its specific MALL, which will vary by drug product.
Davidson and Mangus also examined selected samples of those listed in Table I via measurement of oxygen headspace concentration (OHS) by frequency modulated spectroscopy. A small fraction showed a low level of gas exchange over four weeks. The source of this was not investigated. This observation highlights two points. The first is that systems resultant from certain combinations of components (stoppers, vials, seals) and conditions of assembly (e.g., compression level) may be less robust and thus more likely to be impacted before delivery to the patient (e.g., because of handling/transport or temperature/pressure variation). The second is the merit of evaluating CCI with more than one method—different methods being able to evaluate different sensitivities (this is discussed in detail later).
Performance as a Function of Time
Logistically, a study as a function of time presents a challenge. Consider Table III, which shows commonly used West products and commercial vials. From these, there are 72 permutations of stoppers and vials (note: vial is not a variable as it is determined by stopper, e.g., 13 mm stopper matches only 2 R vial):
4023/50 Gray
(2 configurations) × (2 sizes) × (3 types) × (2 vendors) = 24
4432/50 Gray
(2 configurations) × (2 sizes) × (3 types) × (2 vendors) = 24
4031/50 Gray
(2 configurations) × (2 sizes) × (3 types) × (2 vendors) = 24
Variables in Stopper/Vial Systems. Stoppers are West Products
Ideally, each permutation should be examined. Consider Table IV, which shows the number of samples needed for a one year study with evaluation by He-leak, OHS, and RSF. For 72 permutations, at 330 vials per, 24,000 stopper/vial samples are needed—clearly impractical. As such, a design of experiments (DOE) was developed to establish the landscape of performance aspects with a manageable number of samples.
Sample Count for One-Year Study for a Stopper/Vial Systema
Design of Experiments
The DOE is based on alternating variables—selecting stopper/vial combinations such that each variable is considered with the highest practical number of other variables. It is shown in Table V. Consider, for example, Combinations 1 and 6. It is seen that formulation 4432/50 is considered in two configurations (serum, lyophilization), two sizes (13 mm, 20 mm), with two vial types (EU, Straightwall), and two vendors (Schott, Ompi). This DOE likewise enables examination of performance as a function of compression/RSF and with different measurement methods.
Design of Experimentsa
In Table VI it is shown how many of the possible variables are evaluated. For example, consider the first row (i.e., 4023). It is seen that the DOE enables this formulation to be considered with every other variable. The result of such a study is that a landscape of performance aspects is developed so that trends of performance can be understood. For this DOE, 2600 vial/stopper samples are needed (8 combinations × ∼330 stopper/vial samples per)—admittedly a large number, but manageable as compared to 24,000.
DOE Variables Evaluated
Note that 10 boxes in Table VI are marked “X”, indicating said combination is not evaluated. For example, consider the second row (i.e., 4432) this formulation is not addressed with a vial having US blowback. Over 90% of the combinations are addressed; this is sufficient to describe the performance landscape adequately. Capturing every unevaluated box increases the number of samples substantially and is impractical.
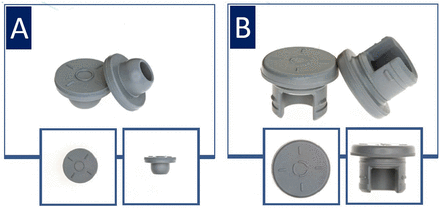
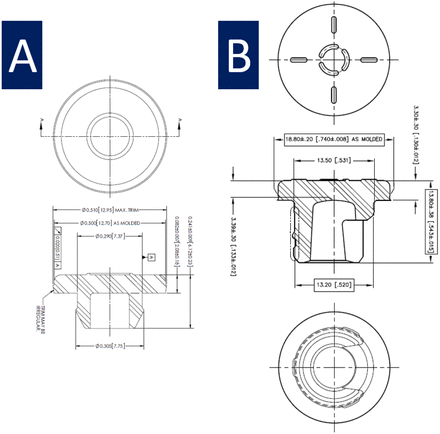
Examples of stopper types and stopper drawings are shown in Figures 1 and 2. Schematic images of vial types are shown in Figure 3.
Stopper types. (A) Serum: 13 mm V-35. (B) Lyophilization: 20 mm S-87-I.
Simplified drawings of stopper types. (A) Serum: 13 mm V-35. (B) Lyophilization: 20 mm S-87-I.
Schematic images of blowback features of vials. (A) Straightwall, (B) European (EU), (C) US.
Materials/Methods
Sample Preparation
Components are listed in Table VII. All stoppers were steam sterilized (121°C for 1 h), followed by drying (105°C for 1 h for serum stoppers, 6 h for lyophilization stoppers). Capping was performed in the ambient environment at Genesis Packaging Technologies (GPT, Exton, PA) using an Integra Laboratory Crimper (three spinning rollers sealing head design). Force was applied to give a target compression followed by activation of the rollers. Three compression levels consistent with typical commercial ranges were set for each combination. Compression levels were measured using a Mitutoyo digimatic indicator, measuring the height of the capped vial before and after crimping; all change is attributed to compression of the stopper because the glass is not compressible.
Components. Stoppers and seals are West products
RSF and CCI Measurements
RSF measurements were made at GPT using an AWG residual seal force tester and analysis algorithms described by Ludwig et al. (8). Initial RSF measurements were made within 5 min of capping. Samples were stored at ambient conditions (23°C ± 3°C) and sent to GPT for RSF measurements at appointed times. Flip-off seal buttons were removed before all RSF measurements. Subsequently, He-leak measurements were made on the exact same samples, after which they were discarded. He-leak measurements were made at West (Exton, PA) using a Leak Detection Associates, LLC SIMS 1284+. Headspace of vials was exchanged with He using thin-gauge needles for entry and exit. Resultant holes were sealed with two-part epoxy. Upon curing, vials were placed in the instrument and a vacuum was applied. The attached mass spectrometer measured flow of He and reported the value as cubic centimeters per second He at standard temperature and pressure (STP; 0°C, 1 atm). All sample vials were filled with He immediately before measurement (e.g., a 3 months sample sat filled with air until measurement). OHS samples were stored in a nitrogen-filled box (100% nitrogen, continuous flow) at ambient temperature (20°C ± 5°C) and returned immediately after measurement (i.e., reused). OHS measurements were made at West using a Lighthouse Instruments LLC FMS-760 Oxygen Analyzer (a frequency modulated spectroscopy method). He-leak, OHS, and RSF measurements were made at the following times: 0, 3 months, 6 months, and 12 months. He-leak and OHS measurements for time 0 were made within 30 days of capping, and within 20 days of subsequent time points. He-leak values and RSF values are averages of 20 samples; OHS values are averages of 30 samples. Sample sizes for RSF and He-leak are those employed typically at West; sample sizes for OHS are those recommended by Lighthouse Instruments, LLC.
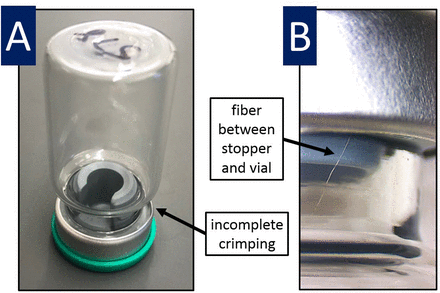
Only four of the 2600 samples examined showed failure by either He-leak or OHS. In all cases, visual inspection revealed the issue to be either a fiber between the stopper and the vial or incomplete crimping, as shown in Figure 4. Data from these were not included in subsequent analyses. Such minor variances are unavoidable because capping was performed under ordinary (i.e., nonsterile) laboratory conditions.
Results and Discussion
Compression levels and initial RSF values are given in Table VIII. Compression levels were targeted to be at, above, and below the values used in commercial applications, that is, nominally 20% compression for 20 mm stoppers and 30%–35% for 13 mm stoppers (7). Compression level was measured immediately after capping (i.e., time 0) only. He-leak data are averages of 20 samples and are reported as: [−log (measured value in cubic centimeters per second at STP)], so that higher numbers correspond to lower leak rates. OHS data are averages of 30 measurements and are reported as percent oxygen. Both He-leak and OHS are deterministic methods endorsed in USP <1207>. RSF data are averages of 20 measurements and reported in pounds. Standard deviations are reported for each.
Compression Levels and Initial RSF Values
He-Leak
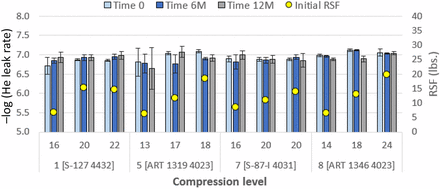
Consider Figure 5. All 20 mm systems show good performance vis-à-vis prior studies (2, 3). However, at lower compressions, Combinations 1 [S-127 4432] and 5 [ART 1319 4023] show a higher level of scatter and slightly higher leak rates. At higher compressions, performance of these is equivalent to other systems. No trend of decrease of performance with time is observed. Further work would be needed to consider why for Combination 1 the RSF value at 22% is not higher; however, general trends are very clear.
He-leak rate performance (cm3/s) of 20 mm stoppers over one year. Details of combinations, e.g., Combination 1 [S-127 4432], are given in Table V. Initial values of compression (percent) and RSF are given. Data at 3 months are not shown. Error bars represent standard deviation. Initial RSF is the value immediately after capping.
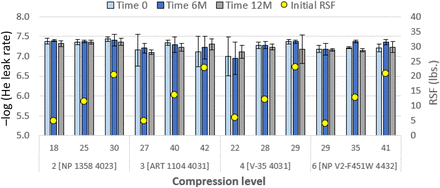
Consider Figure 6. Comparable performance to 20 mm systems is observed for 13 mm systems; all show good performance vis-à-vis prior studies (2, 3). However, at lower compressions, Combinations 3 [ART 1104 4031] and 4 [V-35 4031] show a higher level of scatter and slightly higher leak rates. At higher compressions, performance of these is equivalent to other systems. No trend of decrease of performance with time is observed. Higher scatter and leak rates from lower compressions for select combinations may result from a reduced amount of viscous flow and thus less ability to accommodate vial land seal surface irregularities.
He-leak rate performance (cm3/s) of 13 mm stoppers over one year. Details of combinations, e.g., Combination 2 [NP 1358 4023], are given in Table V. Initial values of compression (percent) and RSF are given. Data at 3 months are not shown. Error bars represent standard deviation. Initial RSF is the value immediately after capping.
Note that for 20 mm systems, the average of all data points is approximately 6.9, whereas for 13 mm systems it is approximately 7.3—indicating on average that more helium is detected from 20 mm stoppers. This is an experimental artifact as the area available through which helium can diffuse through rubber (i.e., vial opening area) is larger for a 20 mm stopper (1.2 cm2) than for a 13 mm stopper (0.38 cm2); a higher rate is expected for 20 mm stoppers.
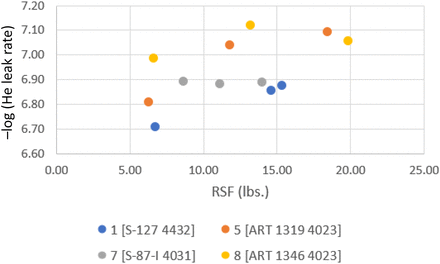
Consider Figure 7. For 20 mm stoppers within initial RSF values of 5–20 lbs., initial He-leak values are within 6.7 and 7.1. This agrees with results reported by DeGrazio. Also in agreement with DeGrazio is the present observation of higher scatter of data with low compression values (low RSF values) (2). This highlights that the holistic approach described, applied at different times/places, enables consistent results.
Initial time He-leak rate performance (cm3/s) of 20 mm stoppers as a function of RSF. Details of combinations, e.g., Combination 1 [S-127 4432], are given in Table V.
OHS
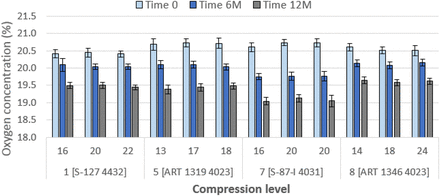
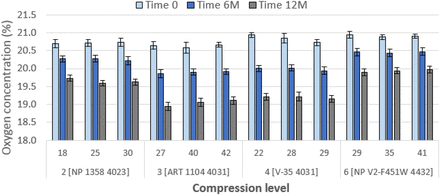
Consider Figures 8 and 9. These show the levels of gas exchange for air-filled vial systems stored under nitrogen. Oxygen levels decrease with time by approximately 1% (from 20.7% to 19.5%). Samples of 4031 (Combinations 3, 4, and 7) show a slightly greater decrease to 19.0%, but as a practical matter, this difference is imperceptible. Clearly, oxygen can permeate through rubber. This is not surprising, as rubber is gas permeable (9). Considered conversely, these data indicate that a halobutyl stopper/vial system assembled in nitrogen and stored in air will contain approximately 1% oxygen after one year.
OHS performance of 20 mm stoppers over one year. Details of combinations, e.g. Combination 1 [S-127 4432], are given in Table V. Initial values of compression are in percent. Data at 3 months are not shown. Error bars represent standard deviation.
OHS performance of 13 mm stoppers over one year. Details of combinations, e.g., Combination 2 [NP 1358 4023], are given in Table V. Initial values of compression are in percent. Data at 3 months are not shown. Error bars represent standard deviation.
Residual Seal Force
RSF may be considered as the force exerted by a compressed stopper against the flange of a vial (10⇓–12). Consider Table VIII and Figures 10⇓–12. As expected, higher compression levels result in higher values of RSF; this is clearly consistent with the literature (6). RSF values decrease within the first 3 months then change asymptotically; this also is clearly consistent with the literature, in particular models described by Zeng and Zhao (12). Under compression, stress relaxation occurs by movement of polymer chains, and RSF values decrease. Buecheler et al. have examined the change in RSF over shorter times (13). Decrease in RSF appeared to have no effect on He-leak or OHS values.
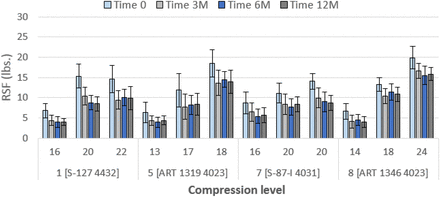
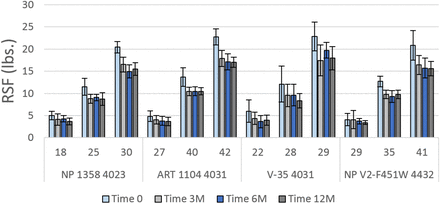
RSF values of 20 mm stoppers over one year. Details of combinations, e.g., Combination 1 [S-127 4432], are given in Table V. Initial values of compression are in percent. Error bars represent standard deviation.
RSF values of 13 mm stoppers over one year. Details of combinations, e.g., Combination 2 [NP 1358 4023], are given in Table V. Initial values of compression are in percent. Error bars represent standard deviation.
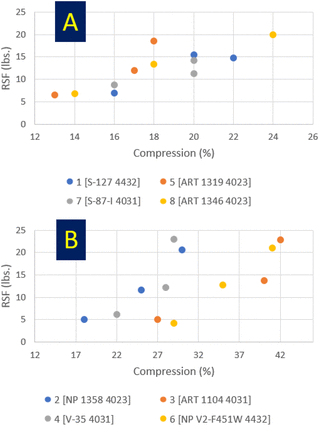
RSF versus compression level: (A) 20 mm stoppers and (B) 13 mm Stoppers. Details of combinations, e.g., Combination 1 [S-127 4432], are given in Table V.
In the present study, RSF values generally did not change in a linear fashion with compression levels. Further investigation would be required. Note the present data set was small—3 compressions per combination—and the compression variation was not large. See Table IX, which cites average compression and variation on average compression for all combinations. Average compression levels range from 16% to 36% (with an average of 24%). Variation on average compression level was only ±21%. With a larger data set and larger variations in compression levels, linear trends might appear. However, it must be kept in mind that results will comprise the composite effect of formulation and stopper configuration.
Compression Level Averages and Variations on Averages
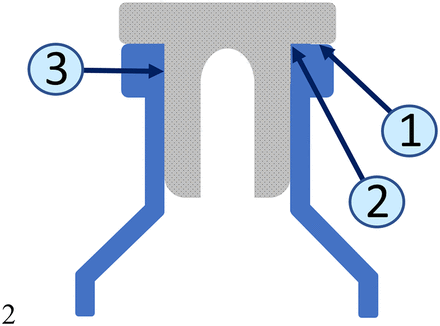
Ovadia et al. have reported that for intact stoppers where 3 sealing surfaces are present (see Figure 13), CCI is independent of RSF (14). Systems with RSF values between 5 N and 20 N (i.e., 1.1 lbs. and 4.4 lbs.) provided good CCI as demonstrated by He-leak values ≤1 × 10−7 cm3/s (i.e., values ≥7.0 on the [−log scale] represented in Figures 5 and 6). However, it was reported that for altered stoppers, comprising only the flange portion (plug portion removed so that only the land seal is present), CCI is dependent upon RSF. For RSF values <20 N, the failure rate increased with decreasing values of RSF. The present results are partly consistent with this observation.
Schematic diagram of a stopper in a vial: (1) land seal, (2) transition seal, (3) valve seal.
At the compression levels and RSF values considered in the present study (which are those that might be used in a commercial application), all systems demonstrated good CCI. Even systems with RSF values as low as 4 lbs. (18 N) demonstrated adequate CCI per Kirsch et al. (see Table II). This observation is made across systems varying in formulation, configuration, size, and vial type. RSF does show a correlation with CCI; lower compression levels (i.e., low RSF values) in select cases, that is, Combinations 1 [S-127 4432], 5 [ART 1319 4023], 3 [ART 1104 4031], and 4 [V-35 4031] gave slightly higher values of He-leak and more variation in values (i.e., larger standard deviations). The practical instruction is that for commercial applications, higher RSF values are preferred. Systems with very low compression levels and corresponding very low RSF values (such as that examined by Ovadia et al.) might show poor CCI; but these were not considered. Optimization of containment system and process are very important.
No system showed any substantive decrease in CCI with time as demonstrated by He-leak measurements. There was some exchange of oxygen in air-filled vials stored in nitrogen as demonstrated by OHS measurements. This was independent of compression level or RSF value. The amount of oxygen permeation was very low but observable. This would have been through the rubber stopper. It is well-known that elastomers, and in fact all polymers, are gas permeable—more so at temperatures above the glass transition temperature (9). This highlights that a system with excellent seal integrity can still permit some exchange (e.g., oxygen, carbon dioxide, water). Systems with very low compression levels and corresponding very low RSF values might display higher rates of oxygen exchange, but these were not considered.
Only one material-related difference was observed—4031/45 (bromobutyl-based) stoppers (Combinations 3, 4, and 7) showed He-leak and RSF performance identical to the performance of 4023/50 (chlorobutyl-based) and 4432/50 (bromobutyl-based) stoppers, but very slightly higher oxygen permeation. This might be expected for a softer (Shore A value of 45 versus 50) material. Comprising a lower filler content and/or a lower level of cross-linking, a softer material might be expected to be less resistant to gas permeability. No other difference was noted based upon formulation, configuration, size, or vial type.
He-leak and OHS results agreed—though OHS clearly revealed the phenomenon of gas diffusion through rubber. This highlights the merit of examining CCI with more than one method.
The present work shows the robust CCI performance of glass–rubber vial systems. However, it is emphasized that most critical is the ability of a system to meet MALL for its drug product; in other words, can the system effectively protect the drug product against anything (e.g., microbial ingress) that would compromise quality. This may involve evaluation of facets beyond the scope of this study, for example, effects on CCI of temperature/pressure variations, or issues resulting from transportation. The holistic approach of DeGrazio serves as an excellent guide to address this (2).
Conclusion
Across a very broad range of components, rubber-glass vial systems showed good CCI performance within the compression levels and RSF values noted. There was no substantial decrease with time or RSF, highlighting the general robustness of rubber-glass vial systems. Lower compression values tended to give slightly lower performance and wider scatter in data, and thus should not be recommended for commercial applications. A small amount of oxygen exchange through stoppers was observed for all systems—to be expected because rubber is gas permeable. This highlights the value of examining performance with more than one method. RSF values decreased initially, then remained constant—consistent with models. Results are consistent with those in the literature. It is emphasized that though CCI methods are instructive, each container system and drug product must be evaluated in terms of meeting the MALL required for that specific drug product and its application. The present results are instructive as part of a holistic approach to assembling a well-performing container system.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgements
Thanks are extended to: (a) the West Analytical Services, LLC team for performance of He-leak and OHS measurements: J. McCaw, B. Jacobs, D. Rowe, T. Vuong; (b) F. DeGrazio for helpful discussions; and (c) Genesis Packaging Technologies for performance of capping and RSF measurements: R. Asselta and C. Flores-Crespo.
FluroTec®, NovaPure®, and Westar® are registered trademarks of West Pharmaceutical Services, Inc., in the United States and other jurisdictions. FluroTec® technology is licensed from Daikyo Seiko, Ltd.
Fiolax® is a registered trademark of Schott AG.
- © PDA, Inc. 2020