Abstract
A drug product is chromatographically screened for organic leachables, derived from the product’s packaging system, as leachables might adversely impact the health of a patient to whom the drug product is administered. Similarly, medical device and packaging system extracts are chromatographically screened for organic extractables as probable leachables. To be protective of patient health, the screening methods must produce recognizable responses for all potentially unsafe substances. To be efficient, the screening methods should provide a means of differentiating between the responses linked to likely to be safe substances and to potentially unsafe substances. The analytical evaluation threshold (AET) was established as a means of differentiating chromatographic peaks, based on concentration, that are unlikely to be unsafe (and thus do not need safety assessment) and that are possibly unsafe (and thus require safety assessment). Thus, the AET manages the competing objectives of protection and efficiency. Although the AET is based on concentration, it is applied based on response. As no chromatographic detection method applied to extractables and leachables screening produces a uniform response to all potential analytes (thus, the magnitude of the response differs across analytes), the objectives of protection or efficiency can be compromised by false negatives and positives. To ensure protection at the expense of efficiency, the AET can be adjusted to address response variation. This article addresses the practical issue that the protectiveness of the AET is affected both by response factor bias and variation and thus correction for only variation is incomplete and ineffective. The article illustrates the proper adjustment of the AET for bias and variation.
Introduction: Extractables, Leachables, and the Analytical Evaluation Threshold
When pharmaceutical drug products are packaged in a container-closure system (CCS), the drug product (DP) and the CCS will chemically interact. Similarly, a medical device interacts with a patient, either directly or indirectly, during its clinical use. One important interaction is leaching, wherein substances (called leachables) originally present in the CCS diffuse into the DP (or substances originally present in the medical device migrate into the patient). Corresponding to leachables are extractables, which are substances that are present in laboratory extracts of the CCS or medical device and which could be leached (i.e., are potential leachables).
The purpose of extractables and leachables assessments is to establish either those extractables that could leach by performing a controlled extraction study on the medical product or to establish those leachables that have leached by performing a migration study. In either case, the extract, the DP, or the contact medium between a medical device and the patient is screened for organic extractables or leachables using chromatographic methods to discover, identify, and quantify these substances. Once the substances have been identified and quantified, this information is interpreted in the context of the effect that the substances could have on the quality of the DP or medical device and/or on patient safety.
An important issue in screening for extractables and leachables is “how low do you go?” That is, one interpretation of the requirements for reporting extractables and leachables is that “you must identify and quantify all substances that are detectable”, which typically means “all peaks with a signal-to-noise ratio greater than 3”. In certain circumstances (such as aggressive extraction of a chemically complex CCS or medical device), this may mean that the number of peaks that need to be reported is large and the difficulty in terms of securing correct identities and accurate quantities for all peaks is significant. In such circumstances, it may be challenging, if not impossible, to accomplish the necessary analytical tasks of identification and quantitation.
To this end, the concept of the analytical evaluation threshold (AET) was developed by an Extractables and Leachables Working Group of the Product Quality Research Institute (PQRI) (1) to facilitate the toxicological safety assessment of extractables and leachables.
Problem Statement: The Analytical Evaluation Threshold Gap
The AET establishes that level at and above which organic leachables in drug products must be reported for toxicological safety risk assessment. In order for a reported leachable to be toxicologically risk assessed, the analytical method must discover the leachable and provide information that leads to the leachable’s identity and concentration in the drug product.
In essence, the AET is a “protective” threshold in the sense that compounds at and above the AET are required to be toxicologically assessed, thereby protecting patient safety.
The AET is calibrated to a well-established toxicological dose threshold (TDT) that has been independently derived considering sound toxicological principles including the proper use of safety factors. The practical reality is that the value of the calibrating dose threshold is established by the duration of the clinical use of the drug product (as well as the drug product’s route of administration). If a parenteral drug product is used chronically, generally defined as cumulative use over a patient’s lifetime of 10 years or more, then the AET is calibrated using the threshold of toxicological concern (TTC) established by ICH M7 (2) for potentially mutagenic impurities, 1.5 µg/day. If the parenteral drug product’s usage is less than chronic, then the AET is calibrated using the U.S. Food and Drug Administration (FDA) accepted toxicological dose threshold for general toxicity and sensitization/irritation, 5 µg/day (3). Similarly, the AET in medical device assessments is calibrated against a dose-based threshold (DBT) that is drawn from ISO/TS 21,726 (4); however, a higher threshold may be considered adequately protective for device contact durations of <30 days (i.e., 120 µg/day).
Armed with the proper toxicological dose threshold, the calculation of the AET is straightforward and requires only that the drug product’s maximum daily dose volume (MDDV) be established. With the MDDV, the AET is calculated as shown in eq 1:
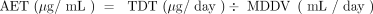 (1)
(1)
For a medical device, the AET tied to a device’s extract is expressed in the context of the conditions under which the extraction is performed:
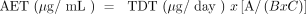 (2)where A is the number of medical devices that were extracted to generate the extract, B is the volume of the extract (measured in mL), and C is the clinical exposure to the medical device (number of devices a user would be exposed to in a day under normal clinical practice).
(2)where A is the number of medical devices that were extracted to generate the extract, B is the volume of the extract (measured in mL), and C is the clinical exposure to the medical device (number of devices a user would be exposed to in a day under normal clinical practice).
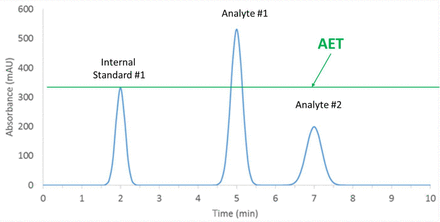
The application of the AET in screening drug products for leachables via chromatography is illustrated in Figure 1. Once the AET is calculated, the drug product is spiked to contain an internal standard, generally at the concentration of the AET. A line at the AET concentration is drawn across the chromatogram using the apex of the internal standard. Peaks whose responses are above or at the line are taken to be present in the drug product at a level greater than or equal to the AET and must be reported for toxicological safety risk assessment. Peaks whose responses are below the line need not be reported for toxicological safety risk assessment as they are deemed to have a negligible adverse effect on patient health and safety.
Pictorial representation of the analytical evaluation threshold (AET). An internal standard has been added to the drug product at a concentration equal to the AET. When the spiked drug product is analyzed, a line at the apex of the internal standard peak is drawn horizontally across the chromatogram. Compounds responsible for peaks whose responses are at or above the line must be reported for toxicological safety risk assessment, for example, Analyte #1. Compounds responsible for peaks whose responses are below the line need not be reported for toxicological safety risk assessment as they are deemed to have a negligible adverse effect on patient safety, for example, Analyte #2. Application of the AET in this manner is based on the assumption that the internal standard and the analytes have the same response factor.
In practice, several variations of this process are employed. Firstly, chromatographic responses are rarely quantified using peak heights and thus the “line in the chromatogram” based on heights is generally replaced by a peak area threshold. Additionally, as the AET varies from situation to situation, it may not be possible to spike the drug product with an internal standard at exactly the AET concentration. In this case, the AET “line” is drawn at some height (response) of the internal standard peak equivalent to the AET.
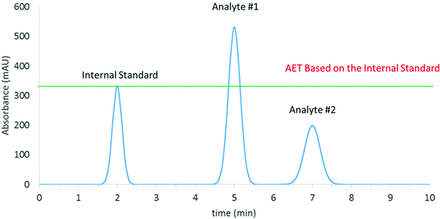
Application of the AET in this manner is predicated on one underlying assumption, that the response factor (RF, eq 3) for every organic leachable (extractable) and internal standard candidate is the same; that is, equal concentrations of leachables and internal standards produce the same magnitude of response. Unfortunately, such a universal response detection method is a goal, as opposed to a reality, with most commonly employed chromatographic detectors. Thus, the simplistic application of the AET as described to this point is prone to error. Specifically, the error is illustrated in Figure 2. There are three possibilities with respect to the relative response of an analyte (leachable) and an internal standard, the trivial case that we have taken previously, which is the RFs of the analytes and the internal standards are equal, and the nontrivial cases in which the RFs of the analytes and the internal standards are unequal. There are two variations to this later case, the circumstance in which the RF of the analyte is greater than that of the internal standard and the circumstance in which the RF of the analyte is less than that of the internal standard.
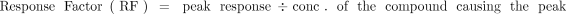 (3)
(3)
Pictorial representation of the analytical evaluation threshold (AET) applied to analytes with different response factors. In this case, the sample contains the internal standard and the analytes at equal concentrations. However, as Analyte #1 has a response factor greater than that of the internal standard, its peak is larger than the peak of the internal standard. Additionally, as Analyte #2 has a response factor less than that of the internal standard, its peak is smaller than the peak of the internal standard. Thus, the application of the AET is complicated. The correct application of the AET in this case is that both Analyte #1 and Analyte #2 need be reported for toxicological safety risk assessment as their concentrations are equal to the AET. However, visual interpretation of the AET would falsely conclude that only Analyte #1 would need to be reported for assessment as the peak is above the AET and that Analyte #2 would not need to be reported for assessment as the peak is below the AET.
As shown in Figure 2, either circumstance creates a problem in applying the AET. In the first circumstance, in which the analyte’s RF is greater than that of the internal standard, the peak for the analyte appears to be greater than that of the internal standard, triggering the false conclusion that the peak is above the AET and therefore that the peak must be reported for toxicological safety risk assessment. In the second circumstance, in which the analyte’s RF is less than that of the internal standard, the peak for the analyte appears to be less than that of the internal standard, triggering the false conclusion that the peak is below the AET and therefore that it need not be reported for assessment.
It is clear that both errors noted previously are a problem. In the first case, an analyte whose RF is greater than that of the internal standard, the problem is one of inefficiency and a potentially excesssive toxicological safety risk assessment, because analytes that do not require toxicological safety risk assessment are nevertheless assessed. In the second case, an analyte whose RF is less than that of the internal standard, the problem is one of underassessing the patient safety risk, because analytes that should be assessed are not being assessed.
Although both of these problems are real and impactful, if one takes the position that “patient safety comes first”, clearly the first problem is a nuisance whereas the second problem is an unacceptable error that must be addressed. Thus, the second problem creates an unacceptable AET gap that must be filled for the toxicological safety of leachables to be fully established.
Filling the Analytical Evaluation Threshold Gap with the Uncertainty Factor
If the root cause of the AET gap is differing RFs among analytes, then an appropriate mechanism for filling the gap is to “correct” or “adjust” the AET for RF variation. One mechanism to produce such an adjustment is through the use of an uncertainty factor (UF), where the UF provides some measure of the magnitude of the RF variation among analytes and internal standards. Mathematically, such a correction to the AET is shown in eq 4:
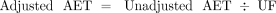 (4)
(4)
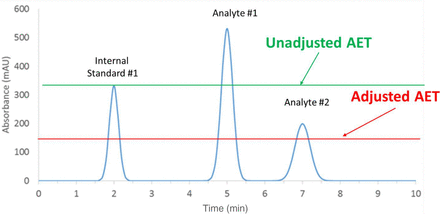
Practically, the use and impact of the adjusted AET is illustrated in Figure 3. By adjusting the AET downward via the UF, analytes with RFs lower than that of the internal standard now have responses that exceed the adjusted AET and thus are properly reported for toxicological risk assessment.
Filling the analytical evaluation threshold (AET) gap. The AET gap is “filled” by adjusting the AET via an uncertainty factor that takes response variation between analytes and internal standards into account. Once the value of the uncertainty factor has been established, the AET is “adjusted” by dividing it by the uncertainty factor. This has the effect of moving the AET line lower in the chromatogram. Thus, Analyte #2, whose response factor is less than that of the internal standard but which is present in the sample at the same level as the internal standard falls into the gap of the unadjusted AET and erroneously is not reported for assessment. However, in the case illustrated here, the AET is sufficiently adjusted by application of the uncertainty factor so that the gap is closed to the extent that Analyte #2 is properly earmarked for assessment.
It is intuitively obvious that to correct for RF variation, the magnitude of the RF variation must be known.
Current Practice with Regard to Establishing the Magnitude of the Uncertainty Factor
The original PQRI recommendation for the AET was that the final (adjusted) AET be an adjustment of an initial (or estimated) AET, with that adjustment being designed to account for analytical uncertainty (RF variation). The specific AET recommendation was “The Working Group proposes and recommends that analytical uncertainty in the Estimated AET be defined as one (1) %Relative Standard Deviation in an appropriately constituted and acquired Response Factor database OR a factor of 50% of the Estimated AET, whichever is greater” (1). Mathematically, this adjustment to produce the final AET can be expressed as (5, 6):
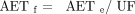 (5)where AETf is the final AET, AETe is the initial (or estimated) AET, and UF is the uncertainty factor.
(5)where AETf is the final AET, AETe is the initial (or estimated) AET, and UF is the uncertainty factor.
In the case of an RF database, the UF has the following value (7):
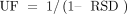 (6)where RSD is the relative standard deviation of the RF database.
(6)where RSD is the relative standard deviation of the RF database.
Current practice in assigning a numerical value to the UF is dictated by the magnitude of the RSD, which varies for the various chromatographic methods used in organic extractables and leachables screening. For screening methods based on gas chromatography (GC), typically coupled with ether mass spectrometric (MS) or flame-ionization (FID) detection, it is generally recognized that the RSD in RF is ≤0.50 (%RSD ≤50%), resulting in a UF of approximately 2, consistent with the PQRI recommendation. For example, in what may have been the basis for the PQRI recommendation, Mullis and associates (7) reported a GC-MS RF database consisting of 32 organic extractables, using either 2-Fluorobiphenyl (2-FB) or p-Terphenyl-d14 (PT14) as internal standards. The statistical analysis of this database is shown in Table I. Statistical analyses of other GC RF databases are summarized in Tables II–V.
Statistical Analysis of a Database of Gas Chromatography-Mass Spectrometry Response Factors Reported by Mullis et al. (8)
Statistical Analysis of a Database of GC-MS and GC-FID Response Factors Reported by Jenke and Odufu (8).a
Statistical Analysis of a Database of GC-MS Response Factors Reported by Zdravkovic et al. (10)a
Statistical Analysis of a Database of GC-MS Response Factors Reported by Christiaens et al. (11)a
Statistical Analysis of a Database of GC Response Factors Reported by Jordi et al. (12)a
A somewhat different situation is encounted with RF for LC screening methods utlizing various detection methods such as MS, ultraviolet (UV) absorption, and charged aerosol (CAD). As noted in Tables VI–IX, RSD values for databases of LC-MS RFs can approach 1, even for databases in which outlier compounds (compounds that have either exceptionally low or high RFs) have been removed from the database.
Statistical Analysis of a Database of LC Response Factors Reported by Jenke and Liu (13)a
Statistical Analysis of a Database of LC Response Factors Reported by Zdravkovic et al. (14)a
Statistical Analysis of a Database of LC-MS Response Factors Reported by Christiaens et al. (11)a
Statistical Analysis of a Database of LC Response Factors Reported by Jordi et al. (12)a
Issues with the Use of Large Uncertainty Factors to Adjust the Analytical Evaluation Threshold
Mathematically, the calculation of the UF via eq 6 and its use to adjust the AET via eq 4 is straightforward. Practically; however, analytical application of the adjusted AET can be quite problematic.
Firstly, there is the effect that RSDs with large values have on the UF. Practically speaking, as the RSD increases past 0.8 (a number that is not unusual for LC-MS RFs), the value of the UF rapidly increases. To a certain point, this escalation in the UF is more a matter of practicality, as the adjusted AET becomes smaller and more difficult to achieve as the UF increases. However, at the point where the RSD = 1, the UF actually loses its physical significance as it first produces an undefined result (when the RSD = 1) and then becomes a negative number (as the RSD becomes >1).
Secondly, and more importantly, there is the practical impact noted previously. As the UF increases and the AET decreases, it can become analytically challenging to achieve the AET with currently available methodology that is expertly applied. To understand this issue, one must remember the purpose of the AET. The AET serves as that threshold at or above which an analyte must be reported for toxicological safety risk assessment. It is intuitively obvious that the first requirement for reporting an analyte is that it be detected, for it is surely the case that before an analyte is identified and quantified it must be detected. Thus, it is a reasonable and necessary circumstance that an analytical method’s limit of detection (LoD) must be less than or equal to the AET. If it is not, the analytical method cannot respond to potentially unsafe analytes above the AET. Because these analytes are not reported, they cannot undergo the required toxicological assessment, and if they cannot undergo the required assessment then the assessment will be incomplete. It is clear that an incomplete assessment means that the analytical methodology is not sufficiently sensitive to be protective of patient health and safety.
Although tangential to the issue of AET adjustment, the preceding discussion brings into play the aspect of the AET’s relationship to method sensitivity, expressed as either the LoD or the limit of quantitation (LoQ). This relationship is mathematically important as the LoD and LoQ are mathematically different quantities. However, from a practical perspective, LoD and LoQ are the same quantity as applied to quantitation in extractables and leachables screening. That is to say that quantitation in extractables and leachables screening is accomplished most typically via use of an internal standard-derived RF, where an analyte’s concentration is estimated as a product of the analyte’s response (peak height or peak area) and the RF. Thus, an extractable’s or leachable’s concentration can be estimated beginning at that concentration at which the peak can first be integrated, which is the LoD. Thus, practically speaking, a screening method’s LoD and LoQ are the same quantity.
The factor that links the AET with the LoD is the drug product’s MDDV, or the extract volume for medical device studies. This is the case as the LoD is a property of the analytical method whereas the AET is a property of the MDDV. As shown in Table X, the larger the MDDV, the lower the AET. The lower the AET, the more likely that the analytical method’s sensitivity (as reflected in the LoD) is inadequate.
Adequate Sensitivity Ramifications of the UF Adjustment to the AET Applied to a Drug Product. Is Method Sensitivity Adequate to Achieve the Adjusted AET?a
This situation would not be all that challenging except for the facts that many drug products have relatively large MDDV values, and that the TDT, which is the basis of the AET, is a small number.
This challenge is illustrated for a drug product in Table X, which considers the circumstance of a chromatographic screening method with an LoD of 0.05 µg/mL (50 ppb). Table X also considers two possible dosing regimens for the drug product, the first being acute use, with an associated TDT of 5 µg/day, and the second being chronic use, with an associated TDT of 1.5 µg/day. In the Table, the unadjusted AET is calculated for MDDV values of 1, 10, and 100 mL. For each one of these cases, the AET is further adjusted with UF values of 2 (representative of GC), 5 (representative of a well performing LC-MS method, and 10 (representative of a “typical” LC-MS method). The question is then asked, “Is the LoD ≤ the AET?” If the answer is “yes”, then the method is sufficiently sensitive and protective of patient safety. If the answer in “no”, then the method is not sufficiently sensitive and is not sufficiently protective of patient safety.
Lastly, Table X summarizes the critical daily dose volume, the MDDV at which the LoD and the AET are equal. At MDDV values below the critical daily dose volume, the method is sufficiently sensitive; at MDDV values above the critical daily dose volume, the method is insufficiently sensitive.
The interpretation of Table X is clear and impactful. As the value of the UF increases to levels required by the high RSD inherent for LC-MS (5 or 10), the MDDV at which the method is no longer capable of achieving the AET gets lower; so low, in fact, that it cannot be achieved for many common classes of drug products. As a worst case, consider, for example, the critical daily dose volume for a chronic drug product whose AET has been adjusted by a UF = 10. This volume, 3 mL, is so small that it excludes almost the entire class of parenteral drug products and many other dosage forms as well.
A similar assessment is shown in Table XI for a medical device. This assessment uses Example B from Appendix section E.4 in ISO 10,993:18 (7), which is based on the scenario of a medical device that is used in a therapy that is completed in 7 days. On each day of therapy, a certain number of devices are required (two devices were specified in the example). In the extraction study, four devices were extracted in 100 mL of extracting vehicle. The resulting extract was neither diluted nor concentrated.
Adequate Sensitivity Ramifications of the UF Adjustment to the AET Applied to a Medical Device. Is Method Sensitivity Adequate to Achieve the Adjusted AET?a
Table XI examines the juxtaposition of the AET and the LoD, taken as 0.05 µg/mL as in the previous example, as a function of the number of devices used per day in the therapy. As noted in Table XI, when 10 devices are used per day and at a UF = 10, the LoD is larger than the AET and the test method is no longer sensitive enough to accomplish its objective. In order to achieve the AET in this circumstance, either the extraction process would have to be changed (extract more devices in a smaller volume) or the extract would have to be concentrated before testing.
These types of assessments clearly and unmistakably indicate the practical effect of high UF values and illustrate the reality that although the adjusted AET can be mathematically calculated for high RSD (and therefore high UF) situations, it does the analytical chemist little good to have an adjusted AET that they cannot achieve. In this case, the AET, whose purpose is to ensure that the analytical chemists focus only on those chromatographic peaks of potential adverse impact, becomes useless as the practical impact of an AET that cannot be achieved is “you must address every recognizable peak in the chromatogram and even when you do you will be missing compounds that you really need to assess”.
Thirdly, there is the practical aspect of the impact of the AET adjustment. To this point in the discussion, we have focused on the positive effect of the adjustment, which is that it provides for the adequate and necessary level of protection for the patients, assuring that all potentially relevant chromatographic peaks are discovered and addressed. However, there is a negative aspect of the adjustment, which is that well-responding compounds whose actual levels are below even the adjusted AET will now require assessment because the AET has been lowered. That is to say that peaks that technically do not need to be reported because their levels are lower than the adjusted AET will need to be reported because, by virtue of their strong response, the peaks will incorrectly appear larger than the adjusted AET. Thus, largely unnecessary effort will be expended to first identify and quantify and then toxicologically safety assess such peaks. In the event that there are difficulties in toxicologically safety assessing the compound of interest (e.g., lack of relevant data), it could be that a potentially safe and clinically important medical product would not become commercialized because of a safety assessment that was overly conservative to account for such difficulties.
Managing the Uncertainty Adjustment
The remainder of this article will focus on managing the necessary requirement that the AET be adjusted so that it is sufficiently protective that it effectively reveals all compounds whose level requires that they undergo toxicological safety assessment, despite their low RF.
Of course, it is important to note that no AET adjustment will address compounds that fail to produce an analytical response.
In the following text we focus on managing the issue, as it is also important to note that the only way to fix the issue of poorly responding compounds failing to be revealed as being above the AET is to develop the almost mystical detection method that is broad in scope, information rich, and invariant in response.
Lastly, the intent of the following discussion is limited to ensuring that the AET in fact reveals all the necessary compounds. Managing difficulties that may arise in the identification and/or quantitation of the revealed substances is a topic for another time.
In the following text, we discuss the proper means of performing the adjustment.
Proper Adjustment of the Analytical Evaluation Threshold
To this point in the article, the AET adjustment has considered the variation in RFs. Although RF variation is certainly an important consideration, it is equally important to consider RF bias.
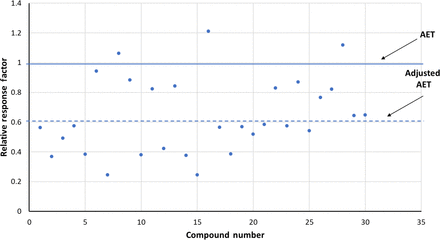
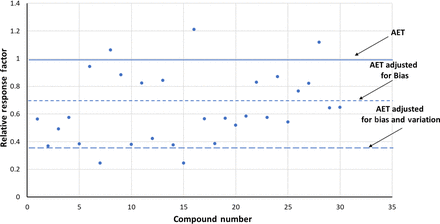
To illustrate this point, Figure 4 shows the distribution of GC-MS RFs reported by Mullis et al. (8), which were described in Table I. Figure 4 illustrates the RFs using 2-FB as the internal standard.
Adjusting the analytical evaluation threshold (AET)—gas chromatography-mass spectrometry response factors. This data set, obtained from Mullis et al. (8) and summarized in Table I, illustrates the situation in which the mean response factor of the data set is significaintly less than 1 (actual mean = 0.642), meaning that a majority of the compounds respond more poorly than does the internal standard. The AET has been adjusted for the relative standard deviation of the database (0.395) via eqs 5 and 6.
In this data set, the mean RF is <1 (actual value 0.642), which means that generally the selected compounds respond more poorly than does the internal standard. The impact of this response bias, compounds versus internal standard, is clearly illustrated by how few compounds fall above the unadjusted AET line. Each one of these compounds in this data set has the same concentration as the internal standard, and because the internal standard is added to the sample at the concentration of the unadjusted AET, each one of these compounds should be revealed by the AET as being appropriate for toxicological assessment. However, as Figure 4 illustrates, only 3 of the 32 compounds in this data set fall above the unadjusted AET and thus are properly revealed.
The RSD of this data set is 0.395 (Table I). Thus, the UF, calculated from eq 6, is 1.65 and the AET is adjusted down to a response value of 1/1.65 = 0.606. This adjusted AET line is also shown in Figure 4. Although such an adjustment does reveal more compounds as requiring toxicological assessment, exactly 50% of the compounds are still below the adjusted AET line, meaning that the adjusted AET fails to properly reveal that these compounds require toxicological assessment.
Of course, the cause of this inability of the adjusted AET to be appropriately protective is the RF bias resulting from the mean RF being markedly below 1.
Thus, proper adjustment of the AET requires two actions: adjustment for the RF bias followed by adjustment for the RF variation. Such a two-step adjustment is illustrated in Figure 5, which uses the same data set as was illustrated in Figure 4.
Properly adjusting the analytical evaluation threshold (AET) by accounting for bias and response variation. This data set, obtained from Mullis et al. (8) and summarized in Table I, illustrates the situation in which the mean response factor of the data set is significantly less than 1 (actual mean = 0.642), meaning that a majority of the compounds respond more poorly than does the internal standard. The AET has been sequentially adjusted for both response bias and response variation.
The first step of the proper AET adjustment is the adjustment for the analytical bias. This adjustment is straightforward; the AET line is moved down to the value of the mean of the RFs for the compounds in the database. As the mean RF in this data set is 0.642, the AET line adjusted for bias is the line drawn at RF = 0.642.
This AET adjusted for bias is then further adjusted for response variation using eqs 5 and 6. As the UF for this data set is 1.65, the AET adjusted for both bias and variation is 0.642/1.65 = 0.389. When the AET line adjusted for both bias and response variation is added to Figure 5, one notes that the fully adjusted AET is highly protective, properly revealing >90% (30 of 32) of the compounds in the data set as requiring toxicological assessment.
It is noted that the “success rate” of the two-step AET in the previous example is not 100%. The practical question here is whether it is reasonable to require that the adjusted AET be 100% protective. Because the AET is based on a TDT that itself is derived via statistical analysis of permissble exposure data, logically it is unreasonable to expect the AET to be 100% protective. However, to the authors’ knowledge, the accptable level of protection has not yet been established in the regualtory or scientific literature.
Two aspects of the two-step AET adjustment require additional comment. Firstly, it is noted that the two-step AET adjustment does not solve the issue of an adjusted AET being so low that it is not analytically viable. In fact, as the two-step adjustment results in lower adjusted AETs, the effect is just the opposite.
The second aspect is whether the two-step process is appropriate for data sets in which the mean RF is >1. Technically speaking, in this case the action taken to generate the AET adjusted for bias would be to move the AET line up to the mean RF. Although this would seem the proper and logical thing to do, it actually has the effect of reducing the adjusted AET’s ability to be protective.
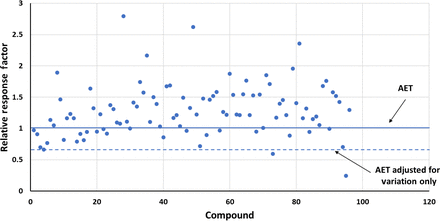
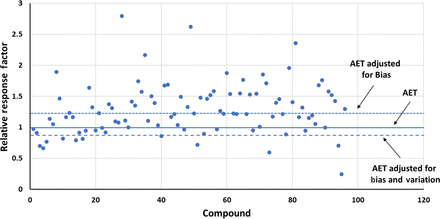
This point is illustrated by considering the GC-MS data set reported by Zdravkovic et al. (10) and shown in Table III. In this database, the mean RF for underivatized compounds was 1.283 and the RSD was 0.323. Figure 6 illustrates the distribution of RFs in this data set and shows the adjustment of the AET for response variation only. In this case, the adjusted AET is highly protective, as only a few compounds in the data set fall below the adjusted AET line. Had the AET been adjusted for both bias (in this case moving the AET line up to the population mean) and variation (using the RSD and eqs 4 and 5), the adjusted AET would be less protective, as nearly 15% of the compounds that should be above the AET line because of their concentration are not above the AET and thus would not be reported for toxicological safety risk assessment (Figure 7).
Adjusting the analytical evaluation threshold (AET)—gas chromatography-mass spectrometry response factors. This data set, obtained from Zdravkovic et al. (10) and summarized in Table III, illustrates the situation in which the mean response factor of the data set is significantly greater than 1 (actual mean = 1.283), meaning that a majority of the compounds respond more strongly than does the internal standard. The AET has been adjusted for the relative standard deviation of the database (0.323) via eqs 5 and 6. Only a few compounds present in the sample at the AET level fall under the adjusted AET and thus are improperly not reported for safety risk assessment.
Adjusting the analytical evaluation threshold (AET)—gas chromatography-mass spectrometry response factors. This data set, obtained from Zdravkovic et al. (10) and summarized in Table III, illustrates the situation in which the mean response factor of the data set is significantly greater than 1 (actual mean = 1.283), meaning that a majority of the compounds respond more strongly than does the internal standard. The AET has been adjusted for both the bias of the method and for response factor variation (using the relative standard deviation of the database (0.323) via eqs 5 and 6). Nearly 15% of the compounds that are present in the sample at the AET level appear to have responses lower than the AET and thus would inappropriately go unreported for safety risk assessment. Thus, in this case, adjusting “up” for response bias is counterproductive from the perspective of protection.
These outcomes lead to the following recommendations in terms of properly adjusting the AET:
If the mean RF of the data set is <1, then the AET is properly adjusted for both response bias and response variation.
If the mean RF of the data set is >1, then the AET is properly adjusted for response variation only.
Conclusion
The AET is a critical and enabling concept in the screening of extracts for organic extractables and drug products for leachables. On one hand, it protects patient safety by ensuring that all potentially unsafe compounds are reported for toxicological safety assessment. On the other hand, the AET ensures that the analytical chemist is not burdened with the responsibility for reporting, identifying, and quantifying every responding compound, regardless of potential safety impact, and that the toxicologist is not burdened with assessing compounds whose adverse effect on patient health and safety is likely negligible. The importance of the AET to the patient is obvious; the importance to the analytical chemist lies in the chemist’s ability to manage the proliferation of chromatographic peaks arising from ofttimes aggressive (and frequently overaggressive) extractions from analytical methods of ever-increasing sensitivity and to address clinical use scenarios that exaggerate a medical product’s typical clinical use in favor of the “one in a million’ situation. This is important to the analytical chemist not only in terms of the number of responses that need to be reported but also in terms of the difficulty of identifying and quantifying small responses likely produced by uncommonly encountered substances. Similarly, this is important to the toxicologist not only in terms of the number of compounds that must be assessed but the difficulty of properly assessing uncommon substances for which there is limited and likely equivocal toxicological data.
Successful application of the AET depends on analytical responses being consistent, compound to compound, a circumstance that is not encountered with the detection methods commonly applied in the chromatographic screening methods for organic extractables and leachables. To remain protective in the face of RF variation, the AET can be adjusted via the use of a UF that is related to the magnitude of the RF variation. Although so doing maintains protection, it comes with the cost of increasing the reporting burden on the analytical chemist and the assessment burden on the toxicologist.
As noted in this article, adjustment of the AET for RF variation is incomplete as it fails to account for another factor affecting the protectiveness of the AET, response bias, which occurs when the AET is based on an internal standard whose response is not the mean of the universe of RFs for extractables and leachables. As demonstrated in this article, the AET becomes both protective and practical when it is properly adjusted for both response variation and bias.
Furthermore, AET adjustment becomes problematic for certain individual analytical methods, such as LC-MS, where RF variation is large and the UF correction becomes challenging, both mathematically and practically.
It is noted that this article considers specifically the aspect of detector response variation. Other sources of experimental variation, including any variation introduced by those processes used to prepare a sample (extract or drug product) for analysis, may be appropriate considerations when addressing total method performance and variation.
Lastly, it is noted that the various databases used and referenced in this article serve the purpose of illustrating the concept of AET adjustment. Given the different circumstances, methods, and purposes by which and for which the databases were generated, it is not appropriate that the databases be compared for the purpose of delineating superior methods or methodologies.
Conflict of Interest Declaration
The authors have no competing interests to disclose; however, Drs. Jenke and Heise note their affiliations with contract research organizations (CROs) that provide extractables and leachables services to the pharmaceutical and medical device industries.
- © PDA, Inc. 2021