Abstract
Successful implementation of continuous processing requires an understanding of how to incorporate viral testing and clearance/inactivation into the process via representative small-scale models. Following the lead of the 2017 Viral Clearance Symposium, a session was devoted to understanding the impact of continuous process conditions on viral safety, how to design process for continuous viral inactivation/removal, and how to leverage existing batch data for a continuous process. In this session, there was a presentation investigating the impact of extended continuous cell culture on the production of endogenous retroviral-like particles, two presentations on the robustness of multicolumn capture chromatography and continuous viral filtration for clearance of viral particles, two talks on leveraging well-characterized batch processing data and scientific knowledge to demonstrate viral clearance capabilities of continuous processing, and finally two presentations related to process designs for continuous viral inactivation. Overall, this session provided additional scientific knowledge to support viral clearance strategies when implementing a continuous manufacturing process.
- Continuous processing
- Downstream processing
- Upstream processing
- Viral Clearance Symposium
- Viral clearance
- multicolumn chromatography
Session Background and Overview
As the biotechnology industry moves toward implementing continuous processing for protein therapeutics, a thorough understanding of the impact that extended processing, dynamic fluid flow, and novel equipment may have on the viral safety of the process is required. To address these concerns, a session focused on discussing viral safety strategies for continuous processing was held at the 2019 Viral Clearance Symposium (VCS). In this session, seven presentations from both industry and Health Authorities demonstrated the robustness of continuous processing unit operations and discussed alternative strategies for viral clearance validation. In the first talk of the session, data were presented that demonstrated how extended continuous cell culture impacts the production of endogenous retroviral-like particles (RVLPs) throughout the duration of the culture period of the specific baby hamster kidney (BHK) cell line. The next two talks discussed the robustness of viral clearance of both multicolumn protein A capture chromatography and integrated continuous viral filtration, with a focus on strategies for how to perform viral clearance validation studies. The fourth and the fifth talks discussed leveraging the scientific knowledge of well-characterized batch processing to engineering models and surrogate viral particles to demonstrate robust viral clearance capabilities in continuous processing. The final two talks focused on the utilization of different process designs for continuous solvent detergent or low pH viral inactivation (VI).
Participants Contributions
Participants were requested to provide a short presentation on recent research projects related to continuous processing. A brief summary of each of these presentations in order of appearance at the symposium is provided.
Study on Endogenous Retrovirus-like Particles in BHK Cells in Continuous Perfusion Process
Shengjiang Shawn Liu, Ph.D., DVM
Bayer Pharmaceuticals LLC, 800 Dwight Way, Berkeley, CA 94710
Endogenous RVLPs in BHK-21 cells were analyzed qualitatively and quantitatively in the development and current good manufacturing practices manufacturing bioreactors for the continuous production of full-length wild-type molecule of recombinant human factor VIII. Morphologically, BHK-21 cells produce dominantly type R endogenous RVLPs (R particles), which were found in the cisternae of the endoplasmic reticulum of BHK cells. Other particles, such as type A particles, were found in 3 of 1800 cells examined (0.20%), whereas C-type particles were not detected in any samples, indicating the BHK-21 cells do not release type C RVLPs naturally into cell culture supernatant. During a 144-day continuous BHK cell culture process, it was found that the cell population capable of producing R particles reached a steady-state RVLP level of approximately 16 particles per cell. However, this cell population was shown to constantly decrease over time, while the R particle-negative cells increased throughout the culture. The reduction of the type R particle-positive cells results in a total RVLP level decrease with time throughout the continuous manufacturing process, indicating that for continuous cell culture, the worst case for RVLP production may occur before the end of the culture run.
Demonstration of Robust Viral Clearance across Two-Column Continuous Protein A Chromatography
James M Angelo1, Srinivas Chollangi1, Thomas Müller-Späth2, Simona Jusyte3, Xuankuo Xu1, and Sanchayita Ghose1
1 Bristol-Myers Squibb, Devens, MA
2 ChromaCon AG, Zürich, Switzerland
3 WuXi AppTech, Inc., Philadelphia, PA
Capture multicolumn chromatography (MCC) is gaining increasing attention lately due to the significant economic and process advantages it offers compared with traditional batch mode chromatography. However, wide adoption of this technology in clinical and commercial space requires scalable models for executing viral validation studies. In this study, viral clearance studies were conducted under current good laboratory practices guidelines to assess mammalian retrovirus (xenotropic murine leukemia virus [X-MuLV]) and parvovirus (murine minute virus [MVM]) clearance across twin-column continuous capture chromatography (CaptureSMB). A surrogate model was also developed using standard batch mode chromatography based on flow path modifications to mimic the loading strategy employed in CaptureSMB. The results showed that cyclical steady-state behavior was achieved by the second cycle for both antibody binding and virus clearance. The surrogate model using batch mode chromatography equipment provided virus and impurity clearance that was comparable with that obtained during cyclical operation of CaptureSMB. This surrogate model was also used to evaluate viral clearance across cycled protein A resin. Further, the log reduction values (LRVs) achieved during CaptureSMB were also comparable with the LRVs obtained using standard batch capture chromatography. Finally, this study also presents our assessments of the resin cleaning strategy during continuous chromatography and how the duration of clean-in-place solution exposure impacts virus carryover (1).
Viral Clearance with Continuous Capture BioSMB and Continuous Viral Filtration
Scott Lute and Sarah Johnson
Center for Drug Evaluation and Research, Office of Biotechnology Products, U.S. Food and Drug Administration, 10903 New Hampshire Ave Silver Spring, MD 20993
The manufacture of biotechnology drug products is ever evolving to increase production and efficiency. The latest trend is a move toward an integrated continuous manufacturing platform that links the upstream cell culture production to the downstream purification. The integration and continuous flow of material between unit operations may change the fundamental way traditional purification techniques are performed, from the use of MCC (i.e., simulated moving bed or periodic counter current) for capture to the application of a dynamic heterogenous product stream on a unit operation such as virus filtration. Acceptance of continuous manufacturing relies on scientific understanding of these unique differences compared with traditional manufacturing. Currently, there are few published articles detailing how to model continuous processes for small-scale validation or what impact the continuous processes may have on viral clearance. In an effort to support the implementation of continuous manufacturing for biotechnology drug products and to provide more scientific understanding, the U.S. Food and Drug Administration (FDA), in collaboration with industry and equipment manufacturers, performed viral clearance research on both simulated moving bed chromatography and continuous viral filtration.
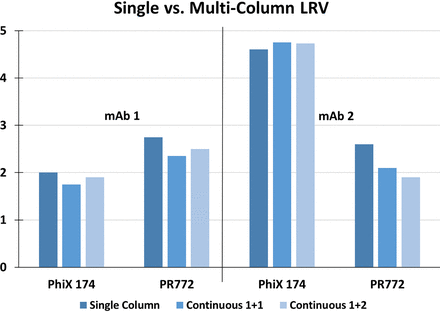
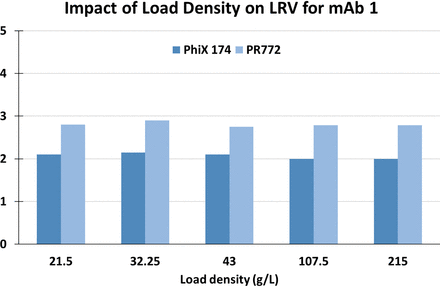
In a collaboration with Pall Corporation and Amgen (2), we evaluated how modifying chromatographic parameters including linear velocity and resin capacity utilization could impact virus clearance in the context of moving from a single column to multicolumn operation. We compared the clearance capabilities between Kaneka Protein A prepacked columns run on a Cadence BioSMB system and on a traditional AKTA Avant system. We found that the viral clearance capabilities of the multicolumn continuous capture system were similar to those of a single column system, and that overloading of a protein A column had no impact on the viral clearance capabilities (Figures 1 and 2, respectively). These data provide scientific support for the use of a single column system as a predictive model for multicolumn capture chromatography.
Comparison of bacteriophage clearance across chromatography platforms from two model mAb feed streams. mAb, monoclonal antibody; LRV, log reduction value.
Overloading a protein A resin has no impact on viral clearance values. mAb, monoclonal antibody; LRV, log reduction value.
In a collaboration with Asahi Kasei (3), we investigated the impact that aspects of continuous viral filtration may have on virus removal capabilities. We first developed small-scale research models for Planova 20 N and Planova BioEX filters to test both extended processing volumes and times, and dynamic product fluid streams. For the extended process models, we were able to successfully run the virus filters for >4 days under low flow rates with no loss in virus load infectivity and no impact on viral clearance capabilities for either filter tested. For the dynamic product fluid model, we successfully used an AKTA Avant to mimic a single spike peak of 10x higher protein, 10x higher salt, or 100x virus spike compared with the normal product fluid stream. We also tested a triple spike as a surrogate for an elution peak from a previous process step being applied to a virus filter. Our research model demonstrated that a single elution peak had no impact on the viral clearance capabilities of the BioEX filter with complete clearance observed in all samples tested. The Planova 20 N filter did have virus passage under a few of the tested conditions. There was no correlation between the spike parameter or filtration pressure and viral passage; however, there was a correlation between total virus loaded and passage, which was previously demonstrated for the Planova 20 N. Overall, we found that continuous processing conditions had minimal impact on the viral clearance capabilities for the Planova 20 N and BioEX filters under our product- and process-specific parameters.
Continuous Virus Validation: Understanding Fundamental Mass Transfer Enables Simplified Virus Validation
Matthew Brown1, Raquel Orozco1, and Jon Coffman1,2
1Boehringer Ingelheim, Fremont, CA, USA; 2Currently AstraZeneca, Gaithersburg, MD, USA
Continuous bioprocessing continues to be at the forefront of therapeutic protein manufacturing innovation. Although more sophisticated continuous downstream unit operations have been developed, there still remains a gap in the industry of a scalable and robust continuous viral inactivation (CVI) solution. Among the challenges that a CVI unit operation faces, a critical parameter that poses a new challenge is the definition of the exact incubation time of the product stream given the dynamic nature of the feed material across operational scales.
Utilizing dye-based pulse injection experiments, the impact of viscosity and CVI reactor scaling parameters on the residence time distribution were tested. This provided a mechanistic elucidation of the flow mechanics occurring within the reactor. The ultimate goal is to demonstrate that residence time in a plug flow reactor is sufficiently comparable to the incubation time of a traditional tank-based operation. This would potentially allow for a simplified batch-based validation strategy (4).
Considerations in Applying Conventional Batch Mode Viral Clearance Data to Continuous Processes
Dan Lacasse
Pfizer, Andover, MA, USA
Continuous integrated purification processes and equipment for biologics may not be readily scaled-down to enable model virus clearance studies. The mechanisms of viral clearance and combined orthogonal clearance requirements for continuous integrated processes would be equivalent to those applied to conventional batch processes. One potential approach to consider for demonstrating viral clearance of an integrated continuous process is evaluation of the clearance of conventional batch unit operations under conditions that bracket the worst-case operational range for the unit operation in the continuous purification process. Potential gaps between the individual viral clearance unit operations and their continuous counterparts, feed stream variance, process controls, and worst-case assumptions will be discussed as part of an approach to sufficiently demonstrate the robust viral clearance capabilities of an integrated continuous purification process.
Process Design and Virus Clearance Studies for Detergent Inactivation in Continuous Processing
Johanna Kindermann1, Thomas Kreil1, Duarte Martins1, Jure Sencar2, and Alois Jungbauer2
1Takeda Pharmaceutical Company Limited, Vienna, Austria
2Austrian Center for Industrial Biotechnology/University of Natural Resources and Life Sciences (ACIB/BOKU), Vienna, Austria
Continuous VI remains one of the missing pieces while the biopharma industry moves toward continuous manufacturing. The challenges of adapting VI to the continuous operation are twofold: 1) achieving fluid homogeneity, and 2) a narrow residence time distribution (RTD) for fluid incubation. To address these challenges, a dynamic active in-line mixer and a packed-bed continuous virus inactivation reactor (CVIR) are implemented, which act as a narrow RTD incubation chamber. The developed concept is applied using solvent/detergent (S/D) treatment for inactivation of two commonly used model viruses. The in-line mixer is characterized and enables mixing of the viscous S/D chemicals to ±1.0% of the target concentration in a small dead volume. The reactor's RTD is characterized and additional control experiments confirm that the VI is due to the S/D action and not induced by system components. The CVIR setup achieves steady state rapidly before two reactor volumes and the LRVs of the continuous inactivation process are identical to those obtained by the traditional batch operation. The packed-bed reactor for continuous VI unites fully continuous processing with very low pressure drop and scalability (5).
Utilizing a Single-Use Two-Tank Viral Inactivation System in a Continuous Downstream Process
Eva Gefroh1, Betsy Barrios1, Tim Wanek1, Lance Horton1, Mike Vandiver1, Mark Brower2, Nuno D.S. Pinto2, Denis Kole3, and Mark Schofield3
1Just Biotherapeutics, Inc., Seattle, WA
2Merck & Co., Inc., Kenilworth, NJ
3Pall Corp., Westborough, MA
A current focus of the biopharmaceutical industry is on intensification of monoclonal antibody processes to achieve higher productivity in smaller footprint systems. Upstream process intensification has focused on high cell density perfusion processes operated over longer durations to increase facility throughput and utilization. For downstream, a multicolumn continuous chromatography process can be coupled to the perfusion culture to capture and purify product. This can be followed by a two-tank VI system to manage the continuous flow. Data are presented on the integrated process and VI system performance, along with a discussion of the system features and readiness for use in manufacturing.
The Pall Cadence VI System is a fully automated, alternating two-tank system that can perform low pH VI on a continuous elution stream. Single-use, gamma-irradiated tubing assemblies and single-use mix vessels are used to maintain fully closed operations. The system relies on the traditional batch approach to VI such that the elution pool is collected to a target volume in the first tank and undergoes batch acid addition, incubation, and base addition in that tank. While the VI steps are being performed in the first tank, elution collection is initiated in the second tank. Elution collection and VI is alternated between the two tanks for multiple cycles over the duration of the continuous process.
An overview of the system by Jones and Schofield (6) discusses the system design to mitigate cross-contamination risk between treated and untreated product. The system is designed to eliminate dead legs within the boundary of the low pH incubation step. Each tank is connected to a recirculation pump that performs the acid and base addition steps but also performs recirculation of the product loop to ensure homogeneity during the low pH incubation. The product recirculation loop has a low point entry back to the tank to eliminate splashing. There is also a dedicated pump and line to transfer neutralized product out of the system. Experiments were performed to verify the system performance, including a riboflavin challenge test to visually confirm the absence of hanging droplets in the mix vessel during operation. Additional challenge tests with the bacterium B. diminuta and bacteriophage Phi6 verified that the system and automated methods for performing the low pH step can effectively inactivate these organisms.
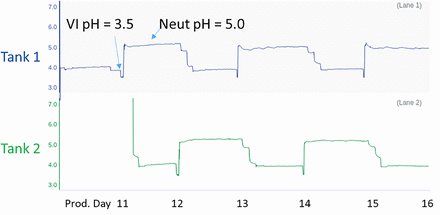
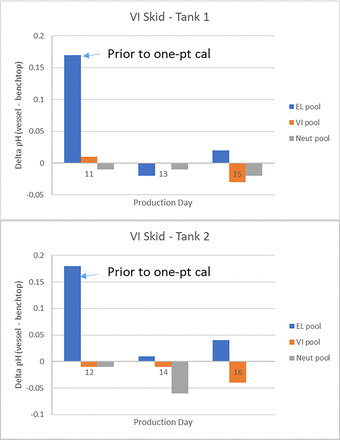
Another critical aspect of the system performance is the accuracy of the pH probe over multiple cycles and days of use, as required for a continuous process. The previously referenced article evaluated pH probe performance over 24 and 48 h using buffer solutions. We evaluated the VI system performance over 6 days in an actual production run, with a continuous perfusion bioreactor at 500 L scale (producing a monoclonal antibody) connected to a continuous capture protein A system followed by the continuous VI system. The elution pool was alternately collected between the two tanks, with an approximately one day cadence between cycles, thus resulting in a total of 6 cycles. Prior to use, Mettler Toledo InPro pH probes were standardized, autoclaved, and aseptically inserted into each mix vessel. Figure 3 shows the cadence of the VI and neutralization cycles in the two tanks, with a VI pH target of 3.5 and neutralized pH target of 5.0. According to the online pH probes, the pH achieved in the VI pool and maintained throughout the VI hold duration was between 3.47 and 3.50; the pH achieved in the neutralized pool was between 5.00 and 5.08. During the run, offline samples were pulled to verify the accuracy of the online pH probe in the mix vessel. Figure 4 shows the difference in pH between the online pH probe in the mix vessel and an offline measurement made with a standardized benchtop pH probe. The pH probes in both mix vessels required a one-point calibration upon first use; however, after that initial calibration, the pH values between online and offline measurements were within ∼0.05 pH units. According to the benchtop pH probes, the pH achieved in the VI pool was between 3.48 and 3.52, and the pH achieved in the neutralized pool was between 5.00 and 5.11. Because the VI pH target is typically set to a ±0.1 range, the accuracy of the titration method and the online pH probes met these criteria. The neutralized pool pH showed a slight overshoot of the target pH; this could be mitigated by increasing the mixing time between base titrant additions in the VI method. The product pools were also monitored for high-molecular-weight (HMW) level by size exclusion chromatography. The results showed the HMW level was 0.1%–0.3% lower in the neutralized pool than the protein A elution pool, indicating that no aggregate generation occurred due to the automated titration methods of the VI system.
VI system pH trends in the two tanks during the 6-day continuous process run. VI, viral inactivation.
Performance of the pH probes in the two tanks during the 6-day continuous process run. Data are presented for multiple stages of the product pool (EL = elution pool, VI = low pH viral inactivation pool, Neut = neutralized pool) shown as the difference in pH measured in the mix vessel and in a representative offline sample measured with a benchtop pH probe.
Although different approaches have been discussed for VI technology in a fully continuous process, there are multiple advantages to using this type of VI system. One is that this system is commercially available and therefore ready for implementation into any continuous process train. Additionally, because it is based on a batch operation, it is expected that the standard regulatory approach of batch spiking for viral clearance validation would be used. The data presented here demonstrate the potential of this system as a robust continuous process solution.
Session Summary
In recent years, the biotechnology industry has showed increased interest in continuous processing as a new manufacturing process or an alternative to a currently licensed manufacturing process. Because of the novel technologies and approaches used in continuous processing, the implementation of a viral safety strategy may not be fully understood from an industry or regulatory perspective. The presentations in the “Continuous Processing” session of the 2019 VCS provided scientific data that support the implementation of continuous VI/clearance unit operations and alternative strategies for viral safety validation in a continuous process.
One aspect of controlling viral clearance in a process is first understanding the amount of endogenous RVLPs produced during the cell culture process. Although this typically occurs in the unprocessed bulk harvest, in a continuous cell culture process, the harvest could be collected daily for an extended period of time. Understanding how RVLP production is impacted by an extended culture duration may help to justify sampling strategies for RVLP enumeration. The first presentation in the session by Bayer Pharmaceuticals provided data demonstrating that RVLP production in BHK-21 cells slowly decreased with time in culture up to 138 days, with peak production around 30 days. The decrease was correlated with either a decrease in RVLP-positive cells in the culture over time or RVLPs disappearing from the cell culture over time. These data were consistent with data in CHO cells presented at the 2017 VCS (7).
The remaining session focused on the understanding of continuous viral removal/inactivation unit operations and alternative validation strategies. Two presentations in this session focused on MCC, a novel technology used for the initial capture unit operation in continuous processing. One of the challenges associated with MCC is understanding how the overloading of columns and rapid column cycling columns could impact the viral clearance capabilities. The presentations by Bristol-Myers Squibb and the FDA demonstrated that MCC with protein A gives comparable viral clearance results to those of batch mode testing (1, 2), and that overloading the column had no impact on viral clearance. These results were observed using two of the major MCC systems commercially available, indicating the robustness of protein A viral clearance with respect to manufacturing mode and equipment as well as that an alternative strategy of using batch mode models for viral clearance of capture MCC is reasonable. The strategy of applying batch mode models to continuous viral clearance validation was continued in the subsequent two presentations by BioPhorum and Boehringer Ingelheim (BI). The BioPhorum presentation discussed the use of batch mode bracketing studies covering the ranges for integrated continuous process unit operations. The results of the bracketing studies in combination with specific unit operation mechanistic understanding of viral clearance may be a feasible approach for continuous viral validation. The BI presentation discussed the use of tracer particles to model mass transfer and flow mechanics for CVI and establish RTDs for various buffer compositions. BI used bacteriophage ΦX-174 to demonstrate that the RTD models successfully predicted viral particle distribution in their Jig in a Box CVI system, supporting the use of minimum residence time and RTD modeling in continuous mode as an alternative viral validation strategy.
In a continuance of the CVI topic, the final presentations discussed the capabilities of two mechanistically different CVI systems. The first system, presented by Takeda, is an incubation chamber consisting of a packed bed of nonporous beads in a column that provides a narrow RTD for continuous S/D inactivation. Data demonstrated comparable clearance values and inactivation kinetics between batch mode and continuous mode for the model viruses X-MuLV and bovine viral diarrhea virus (BVDV). In the last presentation, Just Biotherapeutics demonstrated the robustness of a commercially available CVI system, the Cadence single-use two-tank VI system, for low pH inactivation. The presentation focused on addressing concerns associated with extended processing (e.g., probe accuracy over time) and the use of single-use systems. It was shown that 1) the pH probe had consistent performance over 6 days, 2) the tanks had no hold-up volume in the manifold or mixers (i.e., no detectable bacteria, bacteriophage, or protein in the buffer flush), and 3) HMW species formation was comparable to that of traditional batch mode inactivation, indicating no impact by the fluidic pathway. By demonstrating the comparability and robustness between continuous and batch VI, these presentations support the development and implementation of novel CVI systems on a process-specific basis.
Conclusion
Overall, this session has built on the spirit of innovation observed in the 2017 VCS session on continuous processing. The presenters provided further scientific understanding of the novel continuous processing technologies and presented potential alternative strategies for continuous processing viral validation. Although future work is needed to solidify the robust scientific evidence seen in past batch mode viral validation, this VCS session on Continuous Processing demonstrates that viral validation in a continuous process is achievable.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2022