Abstract
A Contamination Control Strategy (CCS) is a document that focuses on how to prevent contaminations with microorganisms, particles, and pyrogens within sterile and/or aseptic and preferably also in nonsterile manufacturing facilities. This document determines to what extent measures and controls in place are efficient in preventing contamination. In order to efficiently evaluate and control all potential hazards associated with sources of contamination within a CCS, the Hazard Analysis Critical Control Point (HACCP) methodology could be a useful tool to monitor all Critical Control Points (CCPs) related to various sources of contamination. This article describes a way to set up the CCS within a pharmaceutical sterile and aseptic manufacturing facility (GE HealthCare Pharmaceutical Diagnostics) by applying the HACCP methodology. In 2021, a global CCS procedure and a general HACCP template became effective for the GE HealthCare Pharmaceutical Diagnostics sites having sterile and/or aseptic manufacturing processes. This procedure guides the sites through the setup of the CCS by applying the HACCP methodology and helps each site to evaluate whether the CCS is still effective taking all (proactive and retrospective) data following the CCS into account. A summary of setting up a CCS using the HACCP methodology, specifically for the pharmaceutical company GE HealthCare Pharmaceutical Diagnostics Eindhoven site, is provided in this article. Use of the HACCP methodology enables a company to include proactive data within the CCS, making use of all identified sources of contamination, associated hazards, and/or control measures and CCPs. The constructed CCS allows the manufacturer to identify whether all included sources of contamination are under control and, if not, which mitigatory actions need to be performed. All current states are reflected by a traffic light color to reflect the level of residual risk, thereby providing a simple and clear visual representation of the current contamination control and microbial state of the manufacturing site.
Introduction
A Contamination Control Strategy (CCS) is a document that focuses on how to prevent contaminations related to microorganisms, particles, and pyrogens within a sterile and/or aseptic and/or nonsterile manufacturing environment and determines how and to what extent measures and controls in place are efficient in preventing contamination. The principle of a CCS was introduced in the EudraLex—EU guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use—Volume 4, Annex 2 (1). Previously, the International Conference on Harmonisation (ICH) guideline Q10 ha stated the requirement of having a control strategy, defined as a planned set of controls, derived from current product and process understanding that assures process performance and product quality (2). These controls include parameters and attributes related to drug substance and drug products, materials, and components, facility and equipment operating conditions, in-process controls, finished product specifications, and the associated methods and frequency of monitoring and control. The control strategy should facilitate timely feedback, feedforward and appropriate corrective action and preventive action (2). The revised Annex 1 uses the same definition for CCS as the ICH Q10 does but states that the planned set of controls should be specified for microorganisms, pyrogens, and particulates as well (3). These regulatory requirements, as required per ICH Q10 and Annex 1, underline the necessity of using Quality Risk Management to establish a CCS.
Generally, a CCS should be implemented across a Good Manufacturing Practices (GMP)-facility to define all critical control points (CCPs). In addition, a CCS should be in place to assess the effectiveness of all of these controls (such as procedural, technical, organizational, and design) and to monitor measures employed to manage risks associated with contamination. Moreover, the CCS should be actively updated by a cross-functional team on a periodic basis. This document, which can be seen as a holistic assurance of contamination prevention and sterility assurance for sterile products as suggested by Annex 1, should drive continuous improvements concerning control and manufacturing methods (1, 3).
Risk assessment is an important part of the contamination control strategy process and consists of risk identification, risk analysis, and risk evaluation:
During the first step (risk identification), a list of potential hazards related to the source of contamination is defined.
The second step (risk analysis) consists of understanding and estimating the individual parts of the identified risk associated with each identified hazard.
During the third step (risk evaluation), the significance of each source of contamination is assessed by combining individual parts such as a CCP, detection, occurrence, based on the criteria defined during risk initiation (4).
Multiple risk assessment methods can be used for the setup of a CCS, such as Failure Mode and Effects Analysis (FMEA) and Hazard Analysis Critical Control Point (HACCP) methods. FMEA is a more detailed assessment method that is frequently used for complex processes (5). HACCP methodology has been considered to be a risk assessment tool for food safety management. The goal of HACCP is to identify all hazards that are likely to occur in the manufacturing facility through the complete analysis of production processes for all states of the manufacturing chain from manufacturing through to packing and distribution. Additionally, all CCPs throughout the process at which these hazards may be introduced and may cause product impact are identified. The establishment of critical limits for control at those points, the verification of these steps, and the methods by which the manufacturing establishment and quality departments attribute to monitor how well the process control through HACCP is working (6–7).
The HACCP system is based on seven principles, namely:
conducting a hazard analysis,
determining the CCPs,
establishing target levels and critical limit(s) for CCPs,
establishing a system to monitor CCPs,
establishing corrective actions to be taken when monitoring indicates that a particular CCP is not under control,
establishing procedures that verify that the HACCP system is working effectively and as intended and, finally,
establishing documentation concerning all procedures and keeping records effective in line with the current protocols/procedures and their application (8).
By applying all of these principles, all hazards throughout the entire process of manufacturing can be assessed and evaluated.
In order to efficiently evaluate all hazards associated with sources of contamination within a CCS, the HACCP methodology could be useful to monitor all CCPs related to various sources of contamination. It may contribute to providing a complete overview of the entire manufacturing process as well as all CCPs and limits that need to be controlled to ultimately assure that the products comply with the requirements for particles and microbial quality. Additionally, HACCP focuses on how to control these failures. All considerations why HACCP has been applied for this purpose will be discussed within this article. We will also describe the setup of the CCS within a pharmaceutical sterile and aseptic manufacturing facility by applying the HACCP methodology.
Materials and Methods
Setup of a Global CCS Procedure
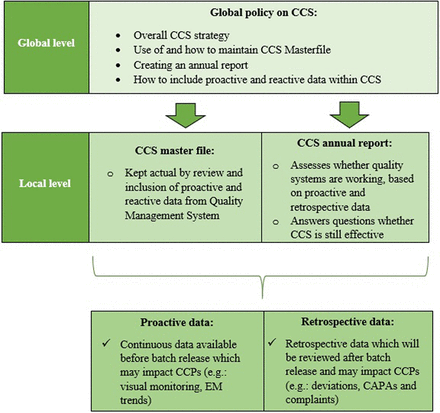
The global CCS procedure—which is a high-level overarching procedure for all sites—contains all general requirements of a CCS, the overall CCS strategy, and how to set up a CCS for each site using a HACCP template (Figure 1). This global CCS procedure also includes a specification of the roles and responsibilities of the site-specific cross-functional team, provides information on how to keep the CCS up to date by using internal (e.g., local standard operating procedures related to microbial control) and external references (e.g., Annex 1 [3] and ICH Q9 [9]), and provides information on how to periodically review the CCS for each site (local level) utilizing a cross-functional team, ensuring compliance with the Annex 1 criterion. Additionally, this global procedure provides information on how the periodic review cycles should be performed and how these cycles enable local sites to efficiently drive continuous GMP-related improvements within the CCS. Finally, this procedure gives information on how to write an annual report, in which it is concluded whether the developed CCS is still effective concerning proactive data (continuous data that might be available within a facility while no product is being manufactured that may impact the different sources of contamination and critical control points, as defined throughout the CCS) and retrospective data (data that will be reviewed after batch release and that are (in)directly related to manufacturing activities and may also influence the different sources of contamination and critical control points, as defined throughout the CCS). Examples of proactive data are clean room classifications, facility design, pressure cascades, and design utilities. Examples of retrospective data include deviations, investigations, spot check trends (visual monitoring), bioburden data, trend analysis data, corrective & preventive actions (CAPAs), audit observations, sterility test results, environmental monitoring (EM) data, complaints, and change controls having an impact on sterility or critical processes.
Schematic overview of global and local level CCS-related documents including proactive and retrospective data. CAPA, corrective & preventive action; CCS, Contamination Control Strategy; EM, environmental monitoring.
Setup of the CCS Document
The CCS cross-functional team from GE HealthCare B.V. Eindhoven, The Netherlands, developed a general HACCP template for the CCS in 2018. This HACCP template was also used by other GE HealthCare Pharmaceutical Diagnostics facilities to complete the CCS after successful piloting of the CCS from the GE HealthCare Eindhoven site. This template is presented in Table I. The HACCP template contains 14 columns in which the complete risk assessment is performed according to the HACCP methodology. The template includes all potential sources of contamination, CCPs, and mitigating actions defined throughout the CCS.
HACCP Template Developed for the CCS at GE HealthCare
Results
After the creation of the HACCP template, the CCS was completed by addressing each category of contamination source (e.g., equipment, EM, and facilities) separately, thereby focusing on one topic at each cross-functional team session. If relevant, existing FMEAs were used to assess potential hazards that need to be included within the CCS.
Setup of a Global CCS Procedure
In 2021, the global CCS procedure became effective within the Pharmaceutical Diagnostics Quality Management System (QMS) after successful piloting of the CCS from the GE HealthCare Eindhoven site. This procedure guides all different sites from Pharmaceutical Diagnostics through the (initial) completion of the CCS applying the HACCP methodology. Secondly, it enables cross-functional teams across sites to evaluate whether the CCS is still effective and taking all proactive and retrospective data following the CCS into account.
Setup of the CCS Document
In Figure 1, a schematic overview of all global- and local-level CCS-related documents, including proactive data (such as clean room classifications and pressure cascades) and retrospective data (such as audit observations, deviations, and CAPAs) is provided.
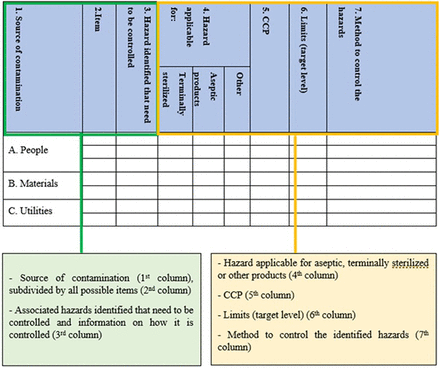
The HACCP template consists of 14 columns in which the complete risk assessment is performed according to the HACCP methodology (Table I). The HACCP-based method for risk assessing sources of contamination and CCPs is one part of the entire CCS, as shown in Figure 1. An overview of the content of all 14 columns is provided following:
Column 1—All potential sources of contamination are defined. These include the following rows: (A) people, (B) materials, (C) utilities, (D) facility design, (E) EM, (F) equipment, (G) processes and outsourced services, and (H) other sources of contamination (Table I).
Column 2—All items related to the specific sources of contamination are mentioned (Figure 2).
Completion of CCS applying HACCP methodology identifying hazards per individual item related to different sources of contamination, and defining critical control points (CCPs), limits and methods to control identified hazards/controls. CCP, critical control point; CCS, Contamination Control Strategy; HACCP, Hazard Analysis Critical Control Point; SOP, standard operating procedure.
Column 3—All associated hazards identified that need to be controlled and information on how they are controlled are included (Figure 2).
Column 4—A distinction is made between filter sterilized, terminally sterilized, or other products (Figure 2).
Column 5—All CCPs are stated per specific item (Figure 2).
Column 6—All limits (e.g., action and/or alert levels) are defined per specific CCP (Figure 2).
Column 7—The method for how to control the identified hazard is given (Figure 2).
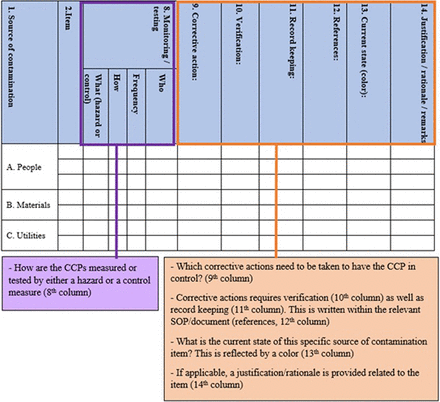
Column 8—It is determined how to monitor (in case of a hazard) or how to test (in case of a control measure) the defined CCPs per specific item. In addition, the frequency of monitoring and the responsible person/department for this approach is included (Figure 3).
Completion of CCS applying HACCP methodology for monitoring/testing all identified hazards/controls and the methodology identifying corrective actions that require verification, documentation, and record keeping by local records and procedures. CCS, Contamination Control Strategy; HACCP, Hazard Analysis Critical Control Point.
Column 9—What corrective actions need to be taken when a CCP is not under control because it exceeds its defined limit (Column 6) (Figure 3).
Column 10—How should verification of these corrective actions take place? (Figure 3).
Column 11—Record keeping in general (Figure 3), that is, the inclusion of recorded fingerprint results in a trend sheet.
Column 12—Local references and/or procedures of the site concerning documentation of the verification steps. (Figure 3).
The HACCP template contains extra columns to indicate the risk profile/state, need for actions and any remarks, if applicable:
Column 13—The current state of each item is highlighted in a specific color in this column (green, orange, or red). Each color reflects the current state and is based on an acceptable residual risk of each item from a contamination control perspective (9). During each review phase of the CCS, the CCS core team decides whether each item is under control (green color), may need risk mitigation or corrective action(s) (orange color), or requires risk mitigation or corrective action(s) (red color), also based on the evaluation of all controls and measures put in place. This is presented in Figures 3 and 4.
Colors (included in the 13th column) highlighting the current state/residual risk of all identified items per source of contamination. CCS, Contamination Control Strategy.
Column 14—If necessary, any justification and/or extra remarks related to the specific source of the contamination item can be added to this column (Figure 3).
Throughout the complete document, the following aspects were included within the CCS in line with the Annex 1: 1. design of both the plant and the processes, 2. premises and equipment, 3. personnel, 4. utilities, 5. raw material controls (including in-process controls), 6. product containers and closures, 7. vendor approval (e.g., sterilization of components and single use systems (SUSs), 8. outsourced services, 9. process risk assessment, 10. process validation, 11. preventive maintenance (equipment, utilities and premises), 12. cleaning and disinfection, 13. monitoring systems, 14. prevention—trending, investigation, CAPA, root cause determination, and the need for more comprehensive investigational tools, and 15. continuous improvements.
Practical Examples Illustrating the Process of CCS Completion Using HACCP Methodology
All possible items and associated hazards that need to be controlled have been defined and categorized per specific source of contamination. This was done using general brainstorm techniques. An example of this is provided in Table II. This example concerns glove contamination within a Grade A and/or B area as a potential source of contamination.
Example of Item ‘Glove Contamination’ as a Potential Source of Contamination within the CCS Applying the HACCP Methodology
For all items, associated hazards as well as control measures were defined. In some cases, both an associated hazard as well as a control measure could be defined for a specific source of contamination. An example of this is presented in Table III.
Example of Item ‘Work Stations within a Grade C Clean Room' and Hazard ‘Contamination of Product by Bioburden on (Critical) Surfaces’ within the CCS Applying the HACCP Methodology
Discussion
This article highlights the setup of a CCS by applying the HACCP methodology to risk assess all potential sources of contamination and CCPs throughout a manufacturing site, which is one part of the entire CCS (Figure 1). The use of proactive risk assessments, such as the HACCP methodology, is encouraged by regulators as part of quality risk management (QRM) philosophies (7, 9). The mapping of all pharmaceutical process flows and steps is crucial to define all QRM elements and to develop process knowledge (10⇓–12).
Evaluation of all risks of contamination within the processes is possible due to identification of all sources of contamination, individual items, CCPs, and all associated hazards and/or control measures within the developed HACCP template. Currently, many risk assessments related to bioburden, sterility assurance, viables, and nonviables are already in place reflecting product quality risk assessment. However, the CCS can be observed as a holistic, high-level document providing a full overview of all contamination control-related process steps and, additionally, reflecting the current state and the residual risks of all of these items regarding the Annex 1 guidelines (1, 9, 13). For example, in the case of glove contamination within an isolator, the CCS will provide not only the CCP (five fingers of glove from operators) and limits (e.g., Grade A < 1 CFU/hand) but also other control actions (risk of contamination or nonsterility of product and risk of exceeding alert and/or alarm level for viables within the isolator) as well as all mitigatory and corrective actions to be taken. As this tool is applicable for all defined sources of contamination, it is very useful to easily assess the impact of contamination control-related issues. The CCS triggers a mitigatory response if specific CCPs are not under control (e.g., when alert or alarm levels are exceeded); it helps a manufacturing facility to adequately respond to the risks and consequences associated with potential sources of contamination. In these cases, associated hazards and/or control measures need to be reflected, and additional mitigating actions need to be performed in line with the CCS. All guidelines and/or documents describing the necessary actions also can be found within the CCS. This proactive risk assessment, therefore, is an ideal and essential tool to assess all sterility assurance-related items impacting a sterile and/or aseptic manufacturing pharmaceutical company.
Multiple risk assessment tools such as FMEA and HACCP have been considered for the setup of a CCS. FMEA is a more detailed assessment method that is frequently used for complex processes (5). It can be used to evaluate potential failure modes of a process (hazard and their impact) and their likely effects on the product or process. Hazard and their impact are identified and ranked by assigning a Risk Priority Number (RPN) to each potential risk based on previously defined scores for detectability, occurrence, and severity (4). To assess the cumulative or combined risks of these failure modes and their effects, FMEA also can be used. Therefore, it is a very useful tool to summarize possible failures, their causes, possible risk-reducing activities as well as their impact on products and processes (4). However, one may argue whether the extensive process of performing a FMEA is the most suitable risk-assessment tool to set up a CCS. Because it would be necessary then to review all RPNs during periodical CCS review, this would delay the whole process compared with reviewing traffic light colors as applied by the HACCP methodology. Therefore, FMEA requires a strong investment in resources and time (14). Additionally, it is also not the most suitable tool to manage residual risks.
Compared with FMEA, HACCP focuses more on CCPs, actions, and/or alert levels and associated hazards/control measures (4). It also focuses on how to control these failures and to manage residual risks. Given the fact that Annex 1 states that a CCS should be implemented across the facility to define all CCPs, and HACCP applies CCPs whereas FMEA does not (1, this underlines the added value for setting up a CCS using HACCP. HACCP methodology does not use RPNs but focuses on hazard control (7). The methodology of not using RPNs but focusing on hazard control was also applied within the CCS of GE HealthCare by using three different colors. These colors were used to reflect whether a particular source of contamination was under control, while no proactive nor retrospective data were reported related to this item within the reviewed time frame (1; green color), or whether risk-mitigating or corrective actions could be implemented because minor but no major issues based on pro- and retrospective data were reported (2; orange color), or whether mitigating or corrective actions must be implemented because one or more major issues were observed based on pro- and retrospective data (3; red color). When no data are readily available, HACCP methodology—focusing on potential hazards and CCPs—is very useful compared with FMEA. Another advantage of HACCP is that it can be used ideally as a prospective risk-assessment tool, thereby enabling inclusion of live data such as pressure cascades and design utilities. Based on these arguments, HACCP was ultimately selected as the risk-assessment tool for the CCS because of its focus on control measures, simplicity, and efficiency.
During each review phase of the CCS, the CCS core team decides which color optimally reflects each individual source of contamination item. This pragmatic approach of applying specific colors to reflect a current state and residual risk of an item instead of assigning RPNs to failure modes contributes to an efficient setup and attributes to effectively maintain a CCS. Although this process of assigning different types of colors to items can be interpreted as a subjective process compared with assigning RPNs, it easily provides insight into the current state of control of all CCPs. Additionally, it will ease the complex process of keeping a CCS up to date, because only the residual risk of each contamination source—reflected by the specific color—has to be evaluated instead of all individual RPNs. A suggestion for companies that already developed FMEAs for sterility-related risk assessments could be to extract failure modes having a high RPN from sterility assurance-related FMEAs to the HACCP-based CCP to adequately assess all associated risks. Such a hybrid model could increase the effectiveness of a CCS. Currently, the pharmaceutical industry highly focuses on retrospective methods and tools, such as root cause analyses. Until now, most experience has been gathered with FMEA to perform risk assessments. Therefore, a limitation might be that pharmaceutical companies have less experience with the application of HACCP methodology. This article provides insights into this methodology, applied for CCS, thereby explaining its applicability as a risk assessment tool for CCS within the pharmaceutical industry. Although retrospective data should also be included within a CCS, these data address problems after they already have occurred instead of preventing them in advance. Proactive data, such as pressure cascades and clean room classifications, are highly important to conclude whether the CCS is still effective and are included within a CCS applying HACCP as a risk assessment tool.
Conclusion
This article summarizes the way a CCS has been developed using the HACCP methodology to risk assess potential sources of contamination and their risks as part of the entire CCS, specifically for the pharmaceutical company GE HealthCare. Using the HACCP methodology for the setup of a CCS enables a company to include proactive data (i.e., continuous data that might be available within a facility while no product is being manufactured, such as design utilities and clean room classifications) and retrospective data (i.e., data that will be reviewed after batch release and which are (in)directly related to manufacturing activities, such as complaints, deviations, and EM data) within the CCS, making use of all identified sources of contamination, associated hazards, and/or control measures and CCPs.
The HACCP methodology still allows other existing risk assessments (e.g., FMEAs) to be used with the HACCP method, especially when hazards need to be further assessed before being included and controlled using the HACCP methodology.
The developed CCS allows the manufacturer to identify whether all of the included sources of contamination are in control and, if not, which mitigating actions need to be performed. All current states and their residual risks are reflected by a color, thereby easily providing a clear representation of the current state with respect to contamination risks of a GMP manufacturing facility.
Conflict of Interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report.
- © PDA, Inc. 2023