Hydroxyapatite and Mixed-Mode Chromatography (HA/MM) Background
Purification processes for antibodies and fusion molecules that contain the Fragment crystallization (Fc) region typically contain protein A and ion exchange chromatographic steps. Occasionally other modes of chromatography are employed to achieve the product quality desired. Hydroxyapatite (HA) is a crystalline form of calcium phosphate that can bind proteins by a mixed-mode ion exchange mechanism. The mechanism of binding is complex and can occur between both the positively charged calcium ions and the negatively charged phosphate ions on the resin matrix. As such the clearance of viruses by HA chromatography is not well understood.
In addition to HA, other mixed modes of chromatography have been increased in their use in purification processes recently. Capto adhere® (GE Healthcare) is a mixed-mode resin with both anion exchange chromatography (AEX) and hydrophobic interaction binding mechanisms. The removal of virus on Capto adhere® is also not well understood. However, it has been shown to be capable of providing substantial clearance of model viruses under certain processing conditions.
Ceramic Hydroxyapatite Chromatography in a Monoclonal Antibody (mAb) Platform Purification Process: Virus Clearance (Eileen Wilson, GlaxoSmithKline)
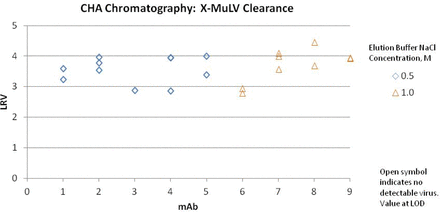
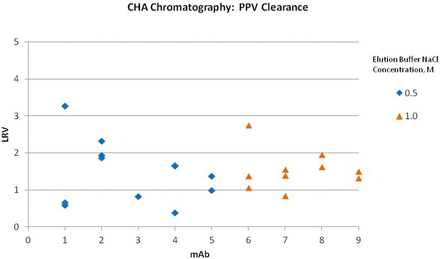
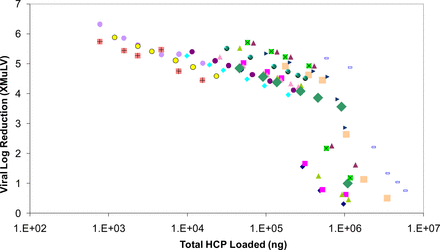
Ceramic HA (Macro-prep Type I) has been used as a polishing step in a platform purification process for mAbs. The step is designed to remove aggregate, host cell protein (HCP), and protein A. The clearance of MuLV and PPV across this step was also evaluated for nine mAbs (Figures 1 and 2). In this platform process, product is bound to ceramic HA and then eluted with an increase in sodium chloride. The buffer matrix, pH, column height, linear flow rate, and residence time were constant across projects. The load samples varied in concentration, but the matrix was similar. Load ratios for the small-scale models were set at the maximum of the production scale, ranging from 23 to 28 mg mAb/mL column volume. The concentration of sodium chloride in the elution buffer was either 0.5 M or 1.0 M, depending on the product.
MuLV clearance by bind and elute ceramic hydroxyapatite chromatography for nine mAbs using Glaxo Smith Kline platform process.
PPV clearance by bind and elute ceramic hydroxyapatite chromatography for nine mAbs using Glaxo Smith Kline platform process.
Some of the earlier studies were done with a single spiked experiment, later studies in duplicate, and some of the original single experiments were later repeated in duplicate. Murine leukemia virus (MuLV) clearance was measured using infectivity assays and porcine parvovirus (PPV) clearance was measured by quantitative polymerase chain reaction (Q-PCR). Studies measured partitioning only, as inactivation was not expected with these chromatography conditions. In all of the MuLV spiking experiments, no virus was measured in the eluate, and the log reduction value (LRV) reported for those experiments is based on the limit of detection. Clearance for PPV was less effective, ranging from 0.4 to 3.3 log10. The sodium chloride concentration in the elution buffer did not correlate with PPV clearance. The ceramic HA step in this platform process does not routinely provide robust PPV clearance. Thus, the origin of this variability remains unknown.
Defining Robust Operating Ranges for Mixed-Mode Chromatography in a mAb Platform Process for Reproducible Viral Clearance (D. Vacante, Johnson & Johnson)
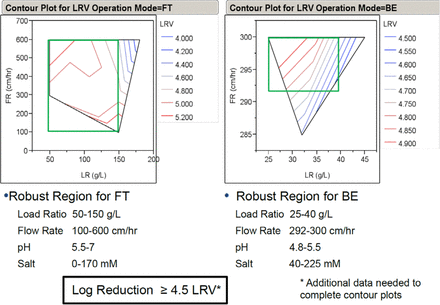
JMP Stepwise Regression Analysis of database parameters were ranked for mixed-mode chromatography showing their relative importance in affecting LRV. Based on the database, a claim of 4.5 log10 was proposed for both Bind and Elute (BE) and flow-through (FT) mode conditions, with ranges determined for protein load ratio, flow rate, pH, and salt concentrations. The following was noted:
BE and FT chromatography modes were analyzed together, but independently, for mixed mode.
Load ratio and flow rate ranked to affect LRV the most, based on regression analysis
Database shows potential for robust clearance of >4.5 log10 for both unit operations
Summary data is presented in Figure 3.
LRV for FT and B/E mode at specific LR, FR, pH, and salt. JMP stepwise regression analysis was conducted for load ratio, flow rate, bed height, absorbance at 280 nm, spike ratio, elution buffer pH and salt concentration, and protein concentration. Plots were generated using an in-house Janssen database containing three mAbs processed in flow-through mode (generating 30 pts) and two mAbs in bind-elute mode (generating 10 pts) with either CaptoAdhere or MMC resins. Here, load ratio and flow rate seem to be the most important parameters for viral clearance.
Virus Clearance on Capto Adhere® (Sherrie Curtis; Genentech, a Member of the Roche Group)
Capto adhere® resin is being evaluated as an alternative resin for purification processes in which Q Sepharose Fast Flow cannot be operated in standard mAb FT mode. Being a mixed-mode resin, with both anionic and hydrophobic functional groups, it has the potential to provide effective impurity clearance including virus removal. Two Capto adhere® processes, a mAb FT mode and a mAb BE, were evaluated using model mAb A, for MuLV and MMV removal.
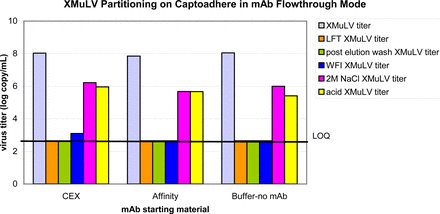
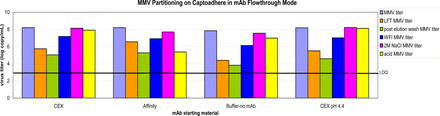
In FT mode, buffer and mAb A in an affinity pool or CEX pool was spiked with either MuLV or MMV at pH 4.0, 3 mS/cm. In these conditions, the mAb flows through the resin. Additional wash phases (water wash, and high-salt wash) were added after the mAb collection to determine if virus is bound via anionic or hydrophobic interactions. In all cases, MuLV bound to the resin while the mAb flowed through as well as during the buffer wash, resulting in highly effective MuLV removal. MuLV remained mostly bound to the resin through the water wash. The most MuLV was found in the high-salt wash and acid regeneration fractions (Figure 4). The majority of MMV that was loaded had bound to the resin; however, MMV was also detected in load, FT, and elution fractions, resulting in only partial MMV removal (2–3 log10). The MMV that had bound to the resin was eluted during water and high-salt washes, and during acid regeneration (Figure 5). The data suggest that Capto adhere® resin can provide effective MuLV and moderate MMV removal.
Partitioning of XMuLV by CaptoAdhere chromatography flow-through mode. CEX (mid-purification) or affinity (post-capture) in-process intermediates were used as starting materials. Grab samples from feedstock and various effluents were quantified by Q-PCR.
Partitioning of MMV by CaptoAdhere chromatography flow-through mode. CEX (mid-purification) or affinity (post-capture) in-process intermediates were used as starting materials. Grab samples from feedstock and various effluents were quantified by Q-PCR.
Key Parameters for MuLV Removal by Capto Adhere® Chromatography (Lisa Connell-Crowley, Amgen)
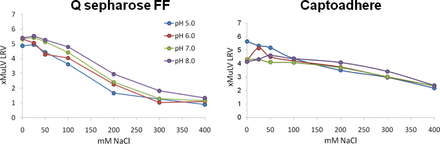
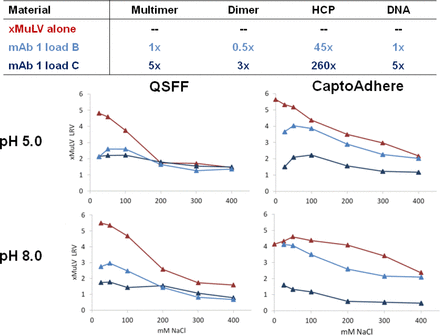
Binding of MuLV to Capto adhere® resin was examined using batch binding high-throughput screening in 96 well plates (see Roush, AEX (Session I) in the same issue). MuLV binding was examined by determining the MuLV LRV in the unbound fraction at pH 5, 6, 7, and 8 and sodium chloride concentrations from 25–400 mM using MuLV Q-PCR. Figure 6 shows that MuLV binding to Capto adhere® is affected by salt level, but not pH, under these conditions. Similar to Q Sepharose Fast Flow (QSFF), binding is improved at lower salt concentrations. Interestingly, MuLV binding to Capto adhere® appears to be more salt-tolerant than binding to QSFF. Figure 7 shows the impact of a mAb load containing two different levels of various impurities, on the binding of MuLV to Capto adhere® as compared to QSFF. For both resins, increased impurity levels significantly reduce MuLV binding. However, at the moderate impurity level (Figure 6, load B), MuLV binding to Capto adhere® appears to be less sensitive to impurity level than binding to QSFF.
Amgen comparison of xMuLV LRV on Q Sepharose Fast Flow and Capto Adhere resins at different pH and salt levels using batch binding HTS. Log reduction value (LRV) was determined by using xMuLV Q-RTPCR on the load and unbound material.
Amgen study of the impact of different mAb loads on xMuLV binding to Q Sepharose Fast Flow (QSFF) and CaptoAdhere.
These data indicate that salt level and impurity level can be key parameters for Capto adhere® chromatography, while pH does not appear to have a significant impact in the range tested. Further experiments identifying impurity(ies) and quantifying the levels responsible for suppressing MuLV binding would be valuable for development and viral clearance validation of a Capto adhere® step.
Adsorptive Membrane Chromatography Background
Membrane adsorbers, a type of chromatography (referred to here as MC) where the matrix is comprised of a filter rather than beads, has been used more extensively in purification processes recently. It offers high throughput and convenient disposability versus conventional column-based chromatography. For mAb processes, MC is typically considered of AEX functionality (i.e., Q-ligands) and is operated in FT mode for the removal of trace impurities. As such, the virus removal mechanism is expected to be similar to AEX, with the majority of virus being removed by binding to the membrane.
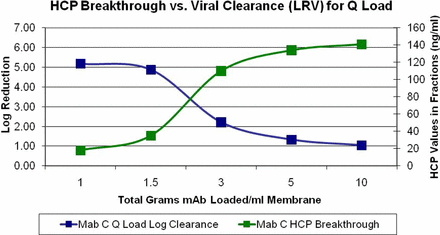
Determination of the Effect of Impurities on Viral Log Reduction across Q Membrane (Judy Glynn, Paul Mensah, and Kristin Thomas; Pfizer)
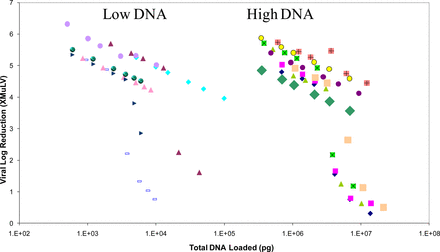
Evaluation of the ability of a Sartobind Q AEX membrane to remove virus when performed in a FT mode showed that in some cases no clearance was obtained for either MuLV or MMV; this loss of clearance appeared to be linked to the presence of a higher level of impurities (host DNA and HCP) in the load material. A series of experiments was performed to determine if the loss of clearance was due to variation in the impurity levels, and whether this relationship could be quantified to ensure adequate clearance in future studies (Figures 8⇓–10).
Pfizer study of the effect of feedstock DNA levels and types (low and high molecular weight) on X-MuLV clearance by Sartobind Q membrane. This scatter plot summarizes multiple experiments.
Pfizer study of the effect of feedstock host cell protein (HCP) levels on X-MuLV clearance by Sartobind Q membrane. This scatter plot summarizes multiple experiments.
Pfizer study of relationship of HCP detected in the flow-through fractions versus viral log reduction.
Experiments were performed utilizing three mAb solutions with varying levels of DNA and HCP in the load. The load material was spiked with MuLV, and fractions of the FT were taken and assayed as representative pools, to determine clearance. The results showed that at a consistent total DNA and HCP level loaded, clearance was reduced and that this amount could be quantified for each impurity, independently. Note that the initial downward trend in log clearance was due to dilution effects; no virus was detected in these fractions. In addition, assays of the individual FT fractions showed that as the relevant impurity began to break through the membrane, clearance began to decline, indicating a relationship between binding the impurity and binding of the virus.
These results can be used to limit the load in future viral clearance studies, in order to ensure that adequate clearance for this unit operation is obtained.
Challenges in Demonstrating Viral Clearance for AEX Membrane Steps (Scott A. Tobler, Morgan Barth, and David Roush; Merck Sharp and Dohme)
Many examples of robust viral clearance have been reported for AEX MC unit operations. In principle, virus behavior with MC should be similar to that in an AEX column chromatography operation, although there are some additional challenges in demonstrating desired levels of clearance. First, MC is typically run to much higher product loadings, and could therefore be more susceptible to impurity levels in the feedstock material (either from the process or the virus spike). In addition, the scale-down versions of MC can have different configurations than the manufacturing-scale modules, which could possibly exhibit both different plugging behavior and different capacities due to under-utilization of the available adsorbent volume.
Recent studies with two similar mAb processes using the Pall Q Mustang membrane have highlighted these challenges. The AEX loads in these processes are around ∼7 g/L mAb, ∼3 mS/cm, and pH 7.5, typical for a mAb process. The operation was designed to effectively reduce HCP and DNA. Because the load is prone to some level of precipitation, a filter train containing 0.5 μm and 0.2 μm pre-filters are used at manufacturing scale in line with the Mustang XT pleated capsule module. The Mustang coin device has been used for the scale-down system; because the pre-filter train cannot be scaled down exactly, a dual-layered filter (0.8/0.2 μm) is used to protect the AEX membrane. Offline filtration of the load material at laboratory scale does not successfully protect the AEX membrane, as small levels of precipitates transiently generated during the run can still foul the membrane (with or without the virus spike). In the viral clearance studies described here, an offline filter control was included to assess viral clearance through this 0.8/0.2 μm pre-filter. Original studies performed without the inline pre-filter resulted in rapid and severe plugging of the AEX membrane, with little or no viral clearance demonstrated.
Table I shows results from follow-up studies using the inline filter for one of the mAb processes (mAb A). LRVs for single runs with MuLV or MMV are shown for three fractions across each run, and they indicate that acceptable clearance was achieved at lower loadings. However, LRVs are reduced at higher loadings, as virus particles were detected in later fractions. Additional studies with both mAb A and mAb B generally showed a trend of high initial LRV, but there was a high variability in the extent of virus breakthrough when loaded to a maximum of 2500 g mAb per L membrane (data not shown).
MMV and MuLV Clearance by Membrane Filtration: Merck, Sharp and Dohme Study Using Fractionation and Inline Filtration
Various experiments were then pursued to investigate whether or not variability in the impurity load during these virus clearance studies might be responsible for the variable and/or low viral clearance results. Table I also shows LRV data for a run using simply the background load buffer matrix spiked with the same MuLV virus. In this run, even in the absence of the product and process impurities, virus breakthrough was observed. A next set of viral clearance runs were performed at a second contract research organization (CRO), using different levels of either virus spike volumes or virus stock purities. Table II summarizes three runs for mAb A spiked with MuLV and shows that, although there is some variability as before, there is no clear difference in breakthrough behavior when spiking a 10 fold range of the virus stock into the load solution. Similarly, for MMV stock preparations with presumably different purities (according to the methodologies described by the CRO), there is no clear difference in LRV for the entire run. Although process stream impurities and a finite capacity of an AEX adsorbent will always be a consideration for the development of this type of step, the results in Tables I and II do not indicate that impurities from either the process, or the virus spike, are responsible for the variability in virus breakthrough that was observed for these processes.
Merck, Sharp and Dohme Study Examining MuLV and MMV Clearance by AEX Membranes Run with Varying Spike Level and Resin Load
More recent runs were performed with an alternative scale-down model for the Mustang Q. This Acrodisc® format still has the normal thickness and number of membrane layers as the coin (each matches the manufacturing-scale modules). However, in the design of this “syringe filter” format, efforts were made to improve flow distribution in order to better utilize the full capacity of the adsorbent. Table III list results for duplicate runs with MuLV and mAb A, and a single run with MuLV and mAb B. All three of these runs showed complete clearance of virus at loads of 1200 g mAb per L membrane.
Merck, Sharp and Dohme Study Examining MuLV Clearance by a New Syringe Filter AEX Membrane Format
It is important to note that although not all of the data are shown here for purposes of brevity; previous studies with duplicate runs using the coin device have always shown variability, even if only loaded to 1200 g/L. Future studies will be performed to determine the reproducibility of viral clearance using a full panel of viruses with this updated version of the scale-down model. However, the preliminary results described here do indicate that a scale-down model with non-ideal flow distribution characteristics could result in variable and/or underestimated LRVs for the MC step.
Case Studies in AEX Filtration for the Removal of Viruses (Michael Clark; Abbott Bioresearch Center, Worcester, MA)
AEX has been proven to be versatile and dependable for the removal of viruses from process streams. The mechanism of action typically involves binding negatively charged viruses while positively charged drug molecules flow through the column. In recent years, a drive to reduce costs and increase the speed and flexibility for early-phase manufacturing processes has led to a switch from reusable AEX resins to disposable AEX membranes, which are fully validated, encapsulated, and intended for single use only.
Based on actual conditions from clinical drug production campaigns, we have compiled several comparative data sets to evaluate MC performance robustness under different operating conditions (Table IV). These include membrane type, loading amount, pH, and conductivity.
Abbott Study Comparing Membrane Filtration Devices with Two Different Antibodies and Three Buffer Compositions. Load Comp, Load Composition; nt, Not Tested; Na Phos., Sodium Phosphate Buffer; NaCl, Sodium Chloride Buffer
The membrane data compilation table shows that the Q Sepharose FF resin, Mustang Q Membrane, Sartobind Q Membrane, and CUNO VR-07 Depth Filter demonstrate robust viral clearance capabilities when loaded under standard conditions (mAb A). In addition, the CUNO VR-07 Depth Filter had reasonably good viral clearance at lower loading amounts.
The following observations were noted:
Over a typical conductivity range of 5–8 mS/cm, MuLV clearance remained consistent and MMV clearance was decreased by 1–2 LRV (mAbs A and B), when evaluated using a Pall Mustang Q membrane.
At load pH values of 6.2 and 8.0, MuLV and MMV removal remained consistent (mAbs C and D), when evaluated using a Sartious Sartobind Q membrane.
Robust viral clearance values obtained for seven different antibodies demonstrated that the ability to remove virus effectively is not directly antibody-related.
Note also that (1) the manufacturing processes described here also used a depth filter (which has hydrophobic interaction and AEX characteristics) as an upstream pre-filter for the Q membrane; the depth filter effectively removes HCPs and DNA from the process stream, which when present are known to reduce viral clearance capability of the Q step (due to competitive binding with virus to the chromatography media), thus allowing the Q membrane to be a designated viral clearance unit operation; and (2) the viral clearance studies used the depth filtrate (pre-Q membrane) for virus spiking and subsequent loading on the Q membranes; results were analyzed by standard plaque assay.
MC Summary
Overall, these data demonstrate that the current generation of AEX membranes can be a robust and cost-effective alternative to AEX resins for the production of drug substance. Given their disposability, they are increasingly being utilized in commercial bioprocessing, particularly for early-stage products where large-scale bead-based chromatography can pose a cost barrier if the possibility exists that resins may be only used once or twice.
UV-C Inactivation and Chemical Precipitation (UV/CP) Background
As bioprocessing technology has evolved, a number of other emerging processing steps are contemplated for downstream processes. These steps have complex mechanisms and are either solely dedicated to virus clearance (e.g., UV-C) or can clear viruses as a side-benefit (e.g., chemical precipitation).
An emerging unit operation specifically devoted to virus inactivation is UV-C. The use of UV-C irradiation to inactivate viruses is well established in other industries, such as drinking water treatment systems. It is believed to inactivate virus genomes and be particularly effective for smaller viruses like parvoviruses, as the penetration depth that the UV-C rays need to traverse is shorter. Application of UV-C to production processes of recombinant biotherapeutic proteins is still in the early stages of being evaluated by biotech firms. One of the concerns of UV-C treatment is that chemical modifications to the protein product may occur at UV-C doses needed for robust viral inactivation. With additional development and refinement, this technology may provide a new, dedicated viral inactivation unit operation that seems to be particularly effective for parvoviruses, which are typically difficult to clear.
Chemical precipitation (CP) of viruses with various additives is another of the new and emerging unit operations being considered for large-scale processing. Unfortunately, the exact mechanism for CP of virus, and the full range of factors that can affect efficiency of virus clearance, is not completely understood. In addition, some of the precipitation agents being evaluated, such as sodium caprylate, may also have viral inactivation properties (1⇓⇓⇓–5). In this case, viral clearance would be achieved by both physical removal and inactivation of the virus, making this a complicated step. Collectively, additional investigation is warranted to fully understand its mechanism.
Evaluation and Development of UV-C Viral Inactivation Processing Methods (Joe Shultz, Amgen)
Amgen investigated the feasibility of a scalable, continuous, flow-through UV-C treatment operation as a viral inactivation method. The previously reported concerns regarding UV-C treatment have been around a balance between the effective dose required to achieve viral inactivation and the potential for UV-C induced modification of the protein product.
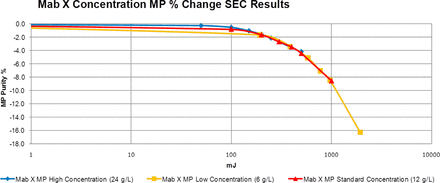
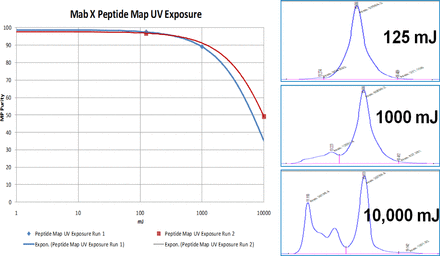
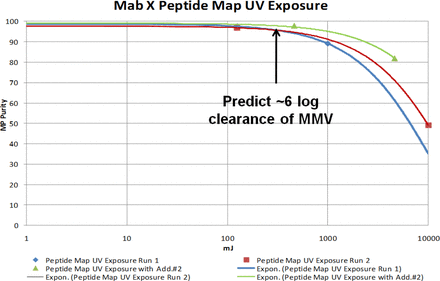
The operation was systematically evaluated with assessments of the solution parameters on UV-C–induced protein modifications, observed UV-C–induced modifications, and evaluation of protein additives to extend dose range. The effects of pH, conductivity, and protein concentration were evaluated across three antibody molecules, as measured by size-exclusion chromatography (SEC) and cation exchange chromatography (CEX) high-performance liquid chromatography (HPLC). There was no discernible pH impact from pH 4.0 to 7.0 or conductivity (data not shown). When a water correction factor was applied to normalize the dose received, after absorbance losses due to the solution constituents, there was no visible effect of protein concentration on the UV-C dose response (Figure 11). However, in all evaluations, changes were seen at UV-C exposure levels above ∼300 mJ.
Effect of solution protein concentration versus corrected UV-C dose received.
Material was produced at high levels of UV-C exposure and analyzed to identify the protein modifications caused by UV-C exposure (Figure 12 and Table V). Extended UV-C exposure results in increased oxidation of methionine and tryptophan residues in the Fc portion of the antibodies. Potency was not affected.
Amgen study of the impact of UV-C exposure intensity on aggregate levels of a model antibody.
Amgen Study Comparing UV-C Exposure versus Oxidation Levels and Activity Trends
A series of protective additives were then investigated to reduce protein modification levels relative to UV-C dose, compared with a control sample. The impact of UV-C–induced protein modification can be significantly reduced with the addition of the protective additives, allowing for increased treatment dosage levels with minimal protein modification. Figure 13 shows the effect of additives on aggregate formation. The dose was adjusted to accommodate for the protein and additive absorbance.
Amgen study of the effect of a protective additive on protein modification response to UV-C dose.
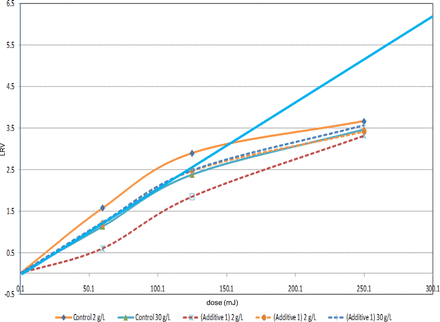
Further, it has been previously suggested that UV-C was not likely to be effective against large enveloped viruses. Therefore, an initial study was performed, with MuLV, to determine the effective dose range required to affect significant viral inactivation. This study was limited by the concentration of virus in the test sample. However, the data showed that >2.5 log10 could be achieved with a 150 mJ UV-C dose and suggested that, if the test sample was not limiting, several log10 could be achieved (Figure 14). Further studies are planned to verify these results and to determine the feasibility of achieving higher LRVs. Finally, there was no significant difference in virus inactivation between samples containing additives and the control without additives. Therefore, it is concluded that the use of protective additives extends the applicable UV-C dose range to those that can potentially provide significant viral clearance, without causing significant protein modification.
UV-C dose versus MuLV inactivation in Amgen study.
It was concluded that UV-C offers the potential to be an effective, large-scale method to inactivate a wide range of virus at high LRV levels. UV-C may provide value as a continuous operation. Potential protein modification needs to be balanced with effective viral clearance, and appropriate additives can extend the range of UV dose exposure to ranges that make this technology feasible as a robust viral clearance unit operation.
Precipitation of MuLV by Caprylate (Lisa Connell-Crowley, Amgen)
Caprylate treatment was previously shown to be an effective method for precipitation of non-immunoglobulin proteins and inactivation of enveloped viruses in plasma-derived intermediates (1⇓⇓⇓–5). A study was conducted to evaluate the clearance of MuLV using caprylate with a purified mAb solution (Table VI). A range of pH 4.9–5.3 was investigated to mimic conditions used for caprylate precipitation of HCPs. Samples containing 15 g/L mAb in 100 mM acetate, pH 4.1 were titrated with combination of 1 M sodium caprylate and 2 M Tris base to desired experimental pH (4.9, 5.1, or 5.3) and final caprylate concentration of 60 mM. In the negative control, the samples were titrated with 2 M Tris base only. Following the titration, virus stock was added to the samples to the concentration of 5% (V/V). Solutions were gently stirred at ambient temperature with time samples taken at 10, 30, 60, and 120 min. At each time point samples were filtered with a 0.45 μm filter to remove potential precipitate that could interfere with analysis by the median tissue culture infective dose (TCID50) method. In order to evaluate possible viral clearance on the 0.45 μm filter, pre-and post-filter samples of 60 min time point were assayed by Q-PCR. Infectivity assay results show complete clearance of MuLV for all conditions tested. However, Q-PCR results suggest that the virus was precipitated by caprylate and consequently removed by the 0.45 μm filter. Similar to HCP precipitation, Q-PCR results show a trend of increasing MuLV removal at higher pH. Further studies would be required to determine if the virus was also inactivated. These data suggest that caprylic acid precipitation can provide a robust and effective viral clearance unit operation, one with a different mechanism than the commonly used low-pH inactivation and virus-retentive filtration.
Amgen Study Evaluating Clearance of xMuLV by caprylate Precipitation from a Purified mAb Solution
- © PDA, Inc. 2014